Table of Contents
Solidification, commonly known as freezing, is a phase transition in which a liquid becomes a solid when its temperature is decreased below its freezing point. The solidification phase shift of a liquid or the liquid content of a material, usually owing to chilling, is defined as freezing by the international definition. Although some authors distinguish solidification from freezing as a process in which a liquid solidifies by increasing pressure, the two concepts are often used interchangeably. The melting and freezing points of most substances are the same; however, certain components have different solid-liquid phase transitions. The melting and freezing limits of agar, for instance, show persistence. It melts at 85 degrees Celsius and solidifies at 32 and 40 degrees Celsius.
A brief outline
The most common method of freezing liquids is crystallization, which involves the development of a crystalline solid from a homogeneous liquid. It’s a first thermodynamic transition phase, which suggests that as much as solid and liquid cohabit, the temperature of the overall system stays very nearly to the melting point due to slow heat removal when in contact with the air, which is a poor heat conductor. The freezing is severely hindered by the latent heat of fusion, and the temperature will not drop any more after the freezing begins but will continue to drop once it is completed. Nucleation and crystal development are the two major events of crystallization.
On a nanoscale scale, “nucleation” is the step in which molecules begin to congregate into clusters, organizing in a specified and periodic fashion that defines the crystal structure. “Crystal growth” refers to the nuclei’s continued growth once they reach the crucial cluster size. The thermodynamics of freezing and melting is a traditional field of physical chemistry that is presently evolving in tandem with computer simulations.
Important concepts
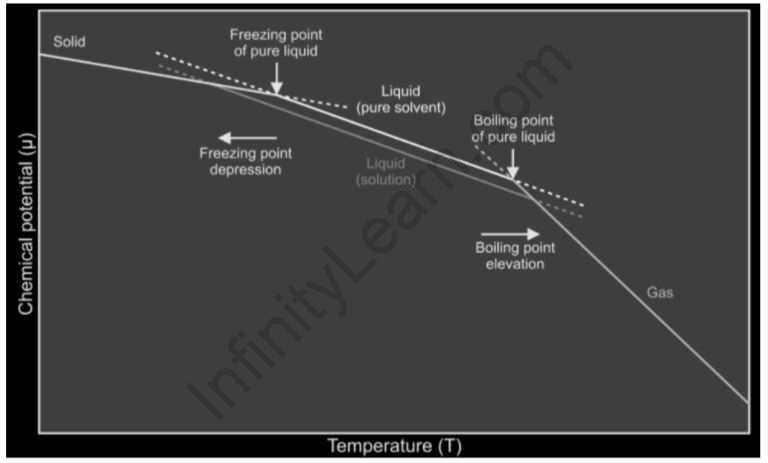
Freezing point depression
The temperature at which something becomes frozen. The addition of solutes lowers the freezing point of solvents, which is referred to as a depression. It’s a colligative feature of solutions that’s proportional to the molality of the solute that’s been added. The following formula can be used to describe a decrease in a solution’s freezing point.
“The vapour pressure of a pure solvent drops with the addition of a solute,” according to Raoult’s law. Because a non-volatile solvent has no vapour pressure, the overall vapour pressure of the solution is lower than that of the pure solvent.
ΔTf = i*Kf*m
Where
ΔTf = Freezing point depression,
I = Van’t Hoff factor,
Kf = Cryoscopic constant, and
m = Molality.
The reason for a solvent’s freezing point being lowered when a solute is added is detailed below.
- There is an equilibrium between both the liquid and solid states of a solvent when it reaches its freezing point.
- This means that the liquid and solid phases have the same vapour pressure.
- The vapour pressure of the solution is found to be significantly lower than the vapour pressure of the pure solvent when a non-volatile solute is added.
- At lower temperatures, the solid, as well as the solution, achieve equilibrium.
Lower freezing point
The freezing point of a substance can be affected by two types of changes: chemical and physical. Mixing a second, soluble component into various liquids lowers their freezing point; this is how road salt stops melt-water from refreezing in cold conditions. Changing the pressure can lower the freezing point of a liquid and produce strange solid forms of a substance that aren’t visible at normal atmospheric pressure.
- When molecules freeze
The temperature at which a substance freezes and boils is determined by electrical interactions between molecules; the stronger the forces, the higher the temperature. Solvent combinations and pressure variations lower the freezing point of liquids by reducing the tensions between molecules.
- Mixing it up
You can lower the freezing point of a liquid by mixing it with another suitable substance. To guarantee complete mixing, the ingredients must be compatible; oil and water, for particular, separate and do not modify the freezing point.
- Taking the pressure off
Pressure changes can cause a substance’s freezing point to rise or fall. Pressures less than one atmosphere lower the temperature with which a substance freezes, yet a higher pressure lowers the freezing point of water.
Molal depression constant
The molality (m) of the solute equals a proportionality constant termed the molal freezing point depression constant has now been calculated to be the freezing point depression of nonelectrolyte solutions. If T0f is the boiling point of the solvent and Tf is the boiling point of the solution, the freezing point depression is calculated as
∆Tf =T0f-Tf
Just like elevation in boiling point, depression in freezing point is also directly related to molality ‘m’.
∆Tf = 1000 x kf x m2 / M2 x m1
Where,
Kf = molal depression constant
m2 = mass of solvent in g
M1 = mass of solvent in kg
M2 = molar mass of solute
Examples of Freezing Points
- Seawater has a freezing point of below 0oC and remains liquid at temperatures below that of pure water. The salts dissolved in it are to blame for this.
- Another example of a solvent’s freezing point being lowered can be found in vodka. It’s an ethanol-in-water solution with a lower freezing point than water but a greater freezing point than pure ethanol.
- Many species can survive in cold climates because their bodies can create chemicals like glycerol and sorbitol, which serve to lower the freezing point of their water bodies.
Significance of depression of freezing point in NEET exam
It would be simpler to get familiar with the thoughts in Chemistry assuming you get ready in a phase interaction and coordinated approach. The depression of freezing point over wretchedness is the most difficult and high-scoring thought in this subject. This subject conveys a great deal of weight. You should seriously mull over the subjective investigation if all else fails in light of the fact that it requires some investment. Our answers furnish you with an exhaustive comprehension of the reasonable subjects. The NEET test was utilized to make Infinity Learn Solutions. Understudies will see that the arrangements are written in simple language and will actually want to accept even the most troublesome ideas.
Also read: Important Topic of Chemistry: Osmotic pressure
FAQs
Is the freezing and softening focuses exactly the same thing?
The edge of freezing over a fluid is the temperature at which it changes into a strong. The softening mark of a strong ought to, in principle, be equivalent to the edge of freezing over of a fluid. Little changes between these amounts can be seen during the activity.
Is it exothermic or endothermic to freeze?
Endothermic cycles incorporate combination, vaporization, and sublimation, while exothermic cycles incorporate freezing, buildup, and affidavit.
At 0 degrees, how rapidly does water freeze?
Whenever water arrives at 32 degrees Fahrenheit (0 degrees Celsius), it freezes, however, the time span it takes relies upon various elements that might contrast from those in your cooler.









