Table of Contents
Osmotic pressure is defined as the pressure required to prevent water from diffusing past a barrier due to osmosis. To put it another way, it relates to how strongly the water would have to “push” through the barrier to distribute to the other side. The concentration of a solute, such as salt, determines osmotic pressure; water will “attempt harder” to diffuse into an area with a high concentration than into one with a low concentration. Osmotic pressure is an expansion of the natural law that all matter would become randomly distributed over time, rather than a “desire” of water to flow.
A brief outline
When the concentrations of compounds in two regions differ but the areas are in close proximity, the random motion of particles causes the substances to diffuse until the solution is homogeneous throughout the entire area. Osmosis describes the process of water traveling across a semi-permeable barrier. As a result, in osmosis, the solutes are unable to move because they are unable to flow through the membrane. Water, on the other hand, can and does travel across the membrane, passing through an area with a higher solute concentration.
Because the internal environment differs from the external environment, this is a critical aspect of biology. Water may flow into or out of cells if the extracellular environment changes. This idea has been used by some creatures, such as plants that employ osmotic pressure to transfer water. Because there’s too much or too little water in the extracellular environment relative to the inside of the cell, it can jeopardize the health of cells and organisms.
Important concepts
Osmotic pressure formula
It is a colligative element that is impacted by the solute molecule fixation in the arrangement. The accompanying equation can be utilized to process osmotic strain:
π = iCRT
Where,
- π = Osmotic pressure
- i = Van’t Hoff factor
- C = molar concentration of the solute in the solution
- R = Universal gas constant
- T = Temperature
Jacobus van’t Hoff, a Dutch physicist, proposed a connection between an answer’s osmotic strain and the molar grouping of its solute. It’s significant that this condition just applies to arrangements that act like amazing ones.
In this case, the solvent molecules would begin to move from the solution side (where the solute levels are high) to the solvent side through the semipermeable barrier (where the solute concentration is low). This is known as reverse osmosis.
Plants depend on osmotic strain to keep their upstanding design. Whenever the plant gets sufficient water, its cells (which contain various salts) retain the water and extend. Plant cells grow, pressing their cell dividers and compelling them to stand erect. At the point when a plant gets deficient water, its cells create hypertonic (they contract because of loss of water). They wither and lose their strength, erect stance accordingly.
Osmosis
The interaction by which dissolvable particles pass from an answer of lower focus to an answer of higher fixation through a semipermeable layer. It is an aloof cycle that happens with practically no consumption of energy.
Osmosis Types
There are two forms of osmosis in general. These are the ones.
- Endosmosis
Water travels into a cell when it is placed in a hypotonic solution, causing it to expand or plasmolyze. This occurs because the solution’s solute concentration is lower than the concentration inside the cell. Endosmosis is the name for this procedure. Endosmosis is the osmosis of a cell or vessel toward its interior. When the aqueous potential outside the cell exceeds the water potential inside the cell, this occurs. As a result, the solute concentration of the surrounding solution is lower than that of the cytoplasm. This type of solution is known as a hypotonic solution. Water molecules flow past the cell membrane and into the cell during endosmosis. Water causes cells to expand as it passes through them.
- Exosmosis
When a cell is placed in a hypertonic solution, the water inside it flows outside, causing plasmolysis (becomes flaccid). This occurs when the concentration of the solute in the solution exceeds the concentration in the cytoplasm. Exosmosis is the name for this procedure.
The osmosis of cells or vessels toward the outside is defined as exosmosis. When the water potential outside the cell is less than the water potential inside the cell, this occurs. As a result, the solute concentration of the surrounding solution is higher than that of the cytoplasm. Water molecules flow out from the cell across the cell membrane, a process known as exosmosis, in hypertonic solutions. The loss of water from cells causes the cells to shrivel.
Osmotic pressure in plants
In many plants, osmotic pressure is the primary source of support. The osmotic entrance of water into a hypotonic plant cell elevates the turgor pressure pushed against the cell wall till the pressure inhibits more water from entering the cell. The plant cells in this micrograph are turgid because their inner vacuoles are full of water.
Osmosis can be extremely destructive to organisms, particularly those without cell walls. If a saltwater fish is placed in freshwater, the fish’s cells will absorb excess water, lyse, and die. The usage of table salt to kill slugs is another example of a detrimental osmotic impact.
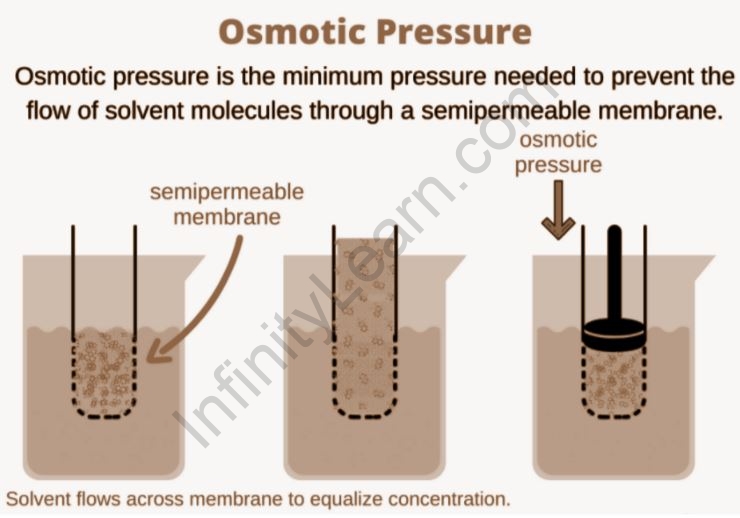
The Importance of Osmosis
- The conveyance of supplements and the release of metabolic by-products are both affected as natural by-products.
- It is accountable for engrossing water from the earth and shipping it to the plant’s higher bits through the xylem.
- This keeps the equilibrium among water and intercellular liquid levels in a living organic entity inside climate stable.
- It holds the bloat of cells under tight restraints.
- It’s a technique for plants to save their water content in spite of standard water misfortune from happening.
- This interaction controls water transport from one cell to another.
- Assimilation causes cell turgor, which controls plant and plant’s part development.
- Without much effort, we are also restricted to eating foods that come from the ground.
- Higher osmotic strain safeguards plants from damage caused by dry season.
Significance of osmotic pressure in NEET exam
This article describes the highly important issue of Osmotic Pressure, which is covered in the chapter solutions. According to the NEET Chapter Wise Weightage for the NEET test, in the osmosis chapter around 2% of the total questions were asked from this chapter throughout the past 33 years. Now that you’ve learned about subject analysis, the next step is to get ready for the questions which have already been asked about these themes in prior papers.
Also read: NEET Exam Pattern 2022
FAQs
Q. What is the variation between osmotic pressure and osmotic potential?
Ans: The hydrostatic pressure that maintains and restricts the osmotic entry of water into a concentrated solution is known as osmotic pressure. Osmotic potential is the ability of a solution to induce water passage into it via a partially permeable barrier as a result of dissolved solutes. Osmotic pressure develops in a closed system, and the result is positive. The osmotic potential, on the other hand, can exist in both closed and open systems and has a negative value.
Q. Osmotic pressure is a colligative feature in what way?
Ans: The osmotic pressure is the least excess pressure required to prevent the solvent from passing through a semipermeable barrier and entering the solution. The amount of solute particle concentration, irrespective of the nature, in relation to the total number of particles in a solution determines the colligative quality of the solution. The osmotic pressure is determined by the number of particles in a solution or its molarity. As a consequence, osmotic pressure is a mutually beneficial feature.
Q. How may osmosis be used to keep food fresh?
Ans: People now use sugar to preserve fruits, and honey is a common preservative to avoid spoilage. Fruit juice or extract typically construct jams and jellies preserved by sugar.Sugar draws water out of food, rendering it unavailable to germs because it generates a hypertonic solution. The loss of water halts microbial activity, causing their death.





