Table of Contents
Once scientists in the seventeenth century started studying the physical properties of gases, they discovered simple relationships between some of the measurable properties of gases. Several scientists established the relationships between the macroscopic physical properties of gases, that is, pressure, volume, temperature, and amount of gas, during the seventeenth and especially the eighteenth centuries, motivated by both a desire to understand nature and a desire to make balloons that could fly. Despite the fact that their measurements were not precise by today’s standards, they were able to determine the mathematical relationships between these variables (e.g., pressure and temperature, pressure and volume) that account for an ideal gas (a hypothetical construct that real gases approximate under certain conditions). Because most moisture content is extracted in the form of water vapour, which behaves as an ideal gas, the ideal gas theory is very important for process analysis. The volume of an ideal gas, the pressure it exerts, and the temperature it has are the three parameters that define it.
The definition of an ideal gas is a special state of matter that can be delimited by a system boundary. In such an ideal gas, particles have no mass and are not attracted to one another; their behaviour can be explained simply by gas molecules colliding with one another and the container’s walls. Real gases, of course, do not exist in an ideal state, but due to their small size and the large amount of space surrounding real gas particles, their size and mass are difficult to measure and are considered irrelevant. As a result, under most conditions, most gases behave more or less “ideally.” Whereas many of these laws apply to ‘ideal’ gases in closed systems at standard temperature and pressure (STP), their principles can still be useful in understanding and altering a significant number of physicochemical processes in the body, as well as drug mechanisms of action.
Overview
In 1811, the Italian scientist Amedeo Avogadro proposed a hypothesis to explain the behaviour of gases, stating that equal volumes of all gases, measured under the same temperature and pressure conditions, contain the same number of molecules. This relationship was supported over time by numerous experimental observations, as expressed by Avogadro’s law. Amedeo Avogadro (1776-1856) proposed one of the most important hypotheses in the development of atomic theory in 1811. At a given temperature and pressure, he proposed that equal volumes of all gases contain an equal number of molecules. This means that a gas’s density—its weight per unit volume—in grams per millilitre must be proportional to its molecular weight. If Avogadro’s ideas had not been dismissed for another 50 years, the process of determining a reliable set of atomic weights for the elements would have taken much less time.
Amedeo Avogadro combined the conclusions of Dalton’s Atomic Theory and Gay Lussac’s Law in 1811 to create Avogadro’s Law, which is an important Gas law. This law gives us an indication of the various properties of gases under different temperature, pressure, volume, and mass conditions. Such laws may seem insignificant, but they play a significant role in our daily lives. The deviation in gaseous behaviour in changed conditions can affect everything from breathing to hot air balloons and vehicle tyres.
Avogadro’s law is defined as the fact that for a given mass of an ideal gas, the amount (number of moles) and volume of the gas are directly proportional, provided the temperature and pressure conditions are constant.
Avogadro’s Law
Avogadro’s law, also known as Avogadro’s hypothesis or Avogadro’s principle, is a gas law. This states that at constant temperature and pressure, the total number of molecules or atoms in a gas is directly proportional to the volume that the gas occupies. Because it connects the temperature, volume, pressure, and amount of substance for gas, this law is closely related to the ideal equation of the gas.
It is known that the number of molecules in 1 mole is known as Avogadro’s Number, and is 6.021023.
Avogadro’s Law Formula
Avogadro’s law can be expressed using the following formula at constant pressure and temperature:
V∝n
V/n=k
Here V is said to be the volume of the gas, n can be used to denote the amount of gaseous substance (in moles), and k is said to be a constant. Whenever the amount of gaseous substance is increased, the corresponding increase in the volume occupied by the gas can be calculated with the help of the following formula:
V1/n1=V2/n2(=k, as per Avogadro’s law ).
Examples of Avogadro’s Law
The respiration is an excellent illustration of Avogadro’s law. When humans breathe in, the molar quantity of air in the lungs increases, as does the volume of the lungs (expansion of the lungs). The image below depicts the change in volume caused by an increase in the number of gaseous molecules.
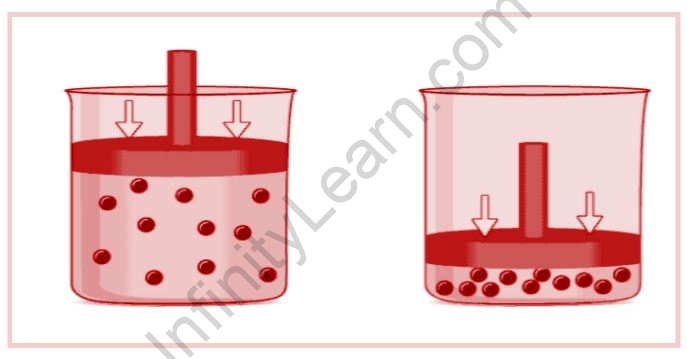
The deflation of automobile tyres is another common application of Avogadro’s law. The number of moles of air present in the tyre decreases as the trapped air inside the tyre escapes. As a result, the volume occupied by the gas decreases, causing the tyre to lose shape and deflate.
Limitations of Avogadro’s Law
Despite the fact that it is perfectly applicable to all ideal gases, Avogadro’s law only provides relationships for real gases. At higher pressures and lower temperatures, the deviation of real gases from ideal behaviour tends to increase. When compared to heavier molecules, gaseous molecules with lower molecular masses, such as hydrogen and helium, follow Avogadro’s law to a large extent.
Also read: Important Topic of Chemistry: Charles’s Law
Frequently Asked Questions
What is Avogadro’s Law in simple terms?
Under the same temperature and pressure conditions, Avogadro's law says that equal volumes of different gases contain an equal number of molecules.
Why is Avogadro’s law important?
Avogadro's law is used to investigate the relationship between the amount of gas (n) and the volume (v). It's a direct relationship, so the volume of a gas is proportional to the number of moles in the gas sample. The law is significant because it allows us to save both time and money in the long run.
Why is Avogadro’s law only for gases?
This is due to a large amount of space between each molecule, which means that the size of the molecule has no effect on the volume of the material. This is why the volume of a gas is determined by the pressure applied to it and why all gases have the same volume when subjected to the same pressure.








