Table of Contents
Introduction
Covalent bonding necessitates a specific orientation between atoms in order to achieve bonding orbital overlap. Sigma-bonding () and pi-bonding () are two types of covalent bonding interactions. Sigma bonds are the strongest type of covalent interaction and are formed by atomic orbitals overlapping along the orbital axis. The shared electrons can move freely between atoms due to the overlapped orbitals. Pi bonds are a weaker type of covalent interaction that occurs when two lobes of the interacting atomic orbitals overlap above and below the orbital axis. Covalent bonds are said to be the strongest bonds in nature and must be broken with the help of enzymes under normal biological circumstances. This really is due to the bonded atoms’ equal sharing of electrons, and as with anything equally shared, there is no conflict to weaken the configuration. Covalent Bond.
The information about covalent bonds from various chemistry-related articles is available here. The covalent bond is an important topic in chemistry. Students who want to flourish in chemistry need to be well known about this to get deep knowledge about it to do well on their exams. The definitions and brief explanations are provided here to assist students in effectively understanding the respective topic. Continue to visit our website for additional chemistry help.
Overview
We can say that a covalent bond is established when electrons from both participating atoms are shared equally. An electron pair involved in this type of bonding is known as a shared pair or bonding pair. It is known that molecular bonds are another name for covalent bonds. The giving of bonding pairs will ensure that the atoms, like noble gas atoms, achieve stability in their outer shell.
A covalent bond, unlike an ionic bond, is stronger between two atoms with similar electronegativity. The bond between atoms with equal electronegativity will be a nonpolar covalent interaction. The electrons in nonpolar covalent bonds are shared equally by the two atoms. For atoms with varying electronegativity, the bond will be a polar covalent interaction in which electrons are not shared equally.
Covalent Bond-What is a covalent bond?
A covalent bond is a chemical link formed between particles by the exchange of electron sets. These electron sets are referred to as shared matches or holding sets, and covalent holding is the consistent balance of alluring and repulsive powers between particles when they share electrons. For some mixtures, electron sharing allows each particle to achieve what is known as a total valence shell, which corresponds to a stable electronic state. Covalent bonds are unquestionably more abundant in nature than ionic bonds.
Covalent Bonding
To form ions, electrons are transferred between atoms of different elements in ionic compounds. However, this is not the only way compounds can be formed. Atoms can also form chemical bonds by sharing electrons equally among themselves. Covalent bonds are the name given to such bonds. Covalent Bonding refers to the forces of attraction or repulsion that exist between two atoms (when they share an electron pair or bonding pair). The electrons which are being shared between the two atoms now wrap around the nuclei of the atoms, forming a molecule. Covalent bonding forms between two atoms when their tendencies to attract electrons are similar (i.e., when both atoms have identical or fairly similar ionization energies and electron affinities).
Covalent bonding encompasses a wide range of interactions, including -bonding, -bonding, metal-to-metal bonding, agonistic interactions, bending bonds, three-center two-electron bonds, and three-center four-electron bonds. In the term “covalent bond”, the prefix co- means “jointly,” “related in action,” “paired to a lesser degree,” and so on; thus, a “covalent bond” means that the atoms share “valence,” as explained in valence bond theory.
Covalent bonding allows the hydrogen atoms in molecule H2 to share two electrons.
Atoms with equal electronegativities have the highest covalency. As a result, covalent bonding does not require that the two atoms be of the same element, only that their electronegativity be equal. Electron sharing between more than two atoms is referred to as delocalized covalent bonding.
Properties:
Only when sharing a single electron pair between atoms does not satisfy an atom’s valence do the atoms share more than one electron pair between them. Some of the covalent bond properties are as follows:
- These bonds are formed between non-metallic elements such as hydrogen and oxygen.
- The generation of new electrons is not the result of covalent bonding. The bond only connects them.
- It can be single, double, or triple bonds, with 2, 4, or 6 electrons shared.
- Atoms can form extremely strong chemical bonds.
- It typically contains about 80 kilocalories per mole (kcal/mol) of energy.
- These bonds rarely break on their own after they are formed.
- Most covalently bound compounds to have relatively low melting and boiling points.
- Covalently bound compounds typically have lower enthalpies of vaporization and fusion.
- Because of the lack of free electrons, covalently bonded compounds do not conduct electricity.
- Water does not dissolve covalent compounds.
Covalent Compounds
Strong intramolecular bonds characterize covalent compounds. This is due to the fact that the atoms within covalent molecules are very tightly bound together. Each molecule is indeed distinct, and the attraction between individual molecules in a covalent compound is typically weak.
We only need a small amount of energy to separate the molecules. This is due to the attractive forces between the molecules, as well as the lack of overall electric charge. At room temperature and pressure, covalent compounds are typically gaseous molecules. They could also be liquids with very low boiling points.
These properties could be attributed to the atoms’ weak intermolecular forces, which hold them together. We do, however, have a large number of solid covalent compounds. Their melting points are low. It is worth noting, however, that a small number of these have a completely different structure. They form massive structures that are held together by a massive number of atoms. Because of the presence of shared electrons, this is possible.
Covalent bonds hold these massive molecular structures together, forming essentially lattices of molecules. These covalent bonds are extremely powerful. They are also very hard and have high melting points, which distinguishes them from most covalent compounds. Diamond and graphite with a carbon atom network are examples of this type of covalent compound. They also include a network of silicon silica and oxygen atoms.
Properties:
- The melting points of covalent compounds are typically low. High melting point molecules such as silica and diamonds are an exception to this rule.
- The boiling points of these compounds are low. This is due to the weak force of attraction that exists between the various bonded atoms. These atoms are held together by Van Der Waals forces.
- These are typically gases or liquids with low boiling and melting points.
- The soft structures of solid covalent compounds are similar to graphite. This is due to the presence of an electron cloud between each layer of carbon atoms.
- Such compounds are not electrical charge conductors. The absence of charged ions is the primary cause of this. Graphite is an exception, as it contains a cloud of electrons. Because of this, graphite is a good conductor.
- They are indeed poor heat conductors. Their molecules are devoid of free electrons, which obstruct the flow of heat energy.
- Polar characteristics are not a general property of covalent compounds. As a result, these compounds are water-insoluble. Water molecules are not completely neutral; The slight negative charge on the oxygen atom and the slight positive charge on the hydrogen atom mean that, since covalent compounds are composed of neutral or slightly charged molecules, they are not strongly attracted to water molecules.
Polar Covalent Bond
Whenever the atoms connected by a covalent bond are not the same, the bonding electrons are shared, but not equally. Instead, the bonding electrons are more attracted to one atom than the other, causing the electron density to shift toward that atom. We define a polar covalent bond by an unequal distribution of electrons and characterize it by a partial positive charge on one atom and a partial negative charge on the other. The atom that attracts electrons the most strongly gains a partial negative charge and vice versa.
Consider this example, electrons in a hydrogen chloride molecule’s H–Cl bond spend more time near the chlorine atom than near the hydrogen atom. As a result, the chlorine atom in an HCl molecule has a partial negative charge, and the hydrogen atom has a partial positive charge. The electron distribution in the H–Cl bond is depicted in the figure.
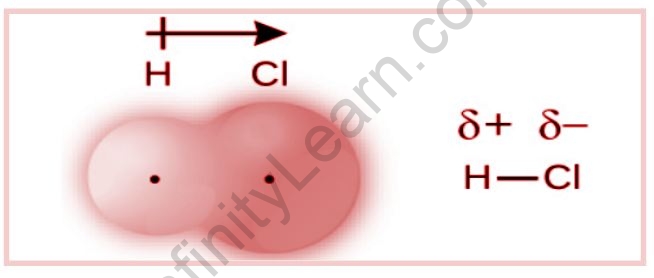
It has been using a lowercase Greek letter “delta,” followed by a plus or minus sign to indicate whether the atom has a partial positive charge (+) or a partial negative charge (–) in a polar covalent bond.
Also read: Valence Bond Theory
Frequently Asked Questions
Q. What is a polar covalent bond?
Ans: When the electronegativity of the joining atoms differ, this type of covalent bond forms, resulting in uneven electron sharing. Iotas that are more electronegative attract electrons. The electronegative difference between the atoms is greater than zero but less than 2.0. As an outcome, the common electron pair gets closer to that particle.
Q. Which compound has both ionic and covalent bonds?
Ans: The compound CaCO3 possesses both ionic and covalent bonds.
Q. Why do Covalent compounds exist as gases, liquids, or soft solids?
Ans: The bond between the atoms in covalent compounds is a strong covalent bond, but the intermolecular forces are weak. Covalent compounds are therefore gases, liquids, or soft solids.









