Table of Contents
The inductive effect, electromeric effect, resonance effects, and hyperconjugation are all electronic variables that impact organic reactions. These electronic parameters affect organic molecules, the majority of which are composed of the six elements carbon, hydrogen, nitrogen, oxygen, phosphorus, and sulfur. Despite this, the restricted number of building blocks does not hinder organic molecules from exhibiting a wide range of physical and chemical properties. Only organic molecules with numerous bonds exhibit the Electromeric Effect. It is a transient effect that occurs when the chemical is exposed to an attacking reagent.
Partial polarity within a molecule causes electron transport among the atoms, resulting in behavior that differs from what would be predicted in a non-polar form of the chemical with no parts that were electron-rich or electron-deficient. Because there is no polarity in the C-C bond and almost no polarity in the C-H bond, saturated hydrocarbons are nonreactive. Because carbon and hydrogen have almost equivalent electronegativity, electrons in a bond between the two atoms are equally attracted to either nucleus and spend roughly the same amount of time orbiting one as the other.
In a non-polar bond, the electron density is uniformly distributed between the two atoms, preventing charged species from attacking or changing the connection. Charged species (electrophiles and nucleophiles, for example) react with polar organic molecules due to an imbalance in electron density or polarity. Higher electronegativity elements, such as oxygen and the halide group, can alter the electron density around an organic molecule, making it more reactive.
Electronic effects can stabilize a molecule, make a complex less volatile, make a molecule more likely to react in a particular manner, or modify the acidity or basicity. Understanding the components involved in electronic imbalance is critical for understanding the underlying mechanics of a chemical reaction, forecasting reaction products, and predicting the behavior of organic molecules.
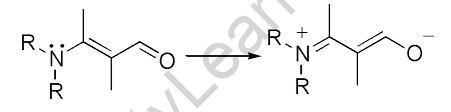
Electromeric Effect
The Electromeric effect refers to the immediate development of a dipole in the molecule of an organic compound as a result of the full transfer of shared pi-electron pairs to one of the atoms under the impact of an attacking reagent. This effect is visible in organic molecules with at least one multiple bond. When an attacking reagent comes into contact with the atoms involved in these multiple bonds, one pi bonding pair of electrons is totally transferred to one of the two atoms.
The electromeric effect is a transient effect that lasts only as long as the attacking reagent is present and in contact with the organic component. When the attacking reagent is withdrawn from the system, the polarised molecule back to its initial state.
Types of Electromeric Effects
The electromeric effect is classified into two types: the +E effect and the -E effect. The direction in which the electron pair is transported is used to categorize it.
-
The +E Effect
When the electron pair of the pi bond is shifted towards the attacking reagent, this result happens. The +E effect may be seen when acid is added to alkenes. The attacking reagent binds to the atom that received an electron pair during the transfer.
When the attacking reagent is an electrophile, the pi electrons are transported towards the positively charged atom, resulting in the +E effect. The protonation of ethene is an example of the +E effect.
2. The -E Effect
When the electron pair of the pi bond is pushed away from the attacking reagent, this result happens. The attacking reagent binds to the positively charged atom in the molecule, that is, the atom that lost the electron pair during the transfer.
When the attacking reagent is a nucleophile, the pi electrons are transferred to the atom with which the attacking reagent will not bind. The addition of nucleophiles to carbonyl compounds is an example of the -E effect.
Electromeric Reaction Mechanism
The mechanism of an electromeric reaction can be described as – When a double or triple bond is attacked by an electrophile E+ (a reagent), the two pi electrons that comprise the pi bond are transferred to one of the atoms. The immediate development of a dipole in the molecule is caused by the transfer of the shared pi electrons. The displacement of the electron pair is depicted by the curved arrow.
Examples of the Electromeric Effect
- In CCl4, an alkene reacts with Br2: Temporary polarization occurs as the reagent bromine approaches alkene, with the C2 atom obtaining a negative charge and the C1 atom gaining a positive charge. The electrophile Br+ attacks alkenes, producing a cyclic bromonium ion as an intermediary. The cyclic bromonium ion is then attacked by Br, yielding vicinal dibromide.
- Hydrogen halide addition: Hydrogen halides contain an electrophile (proton) as well as a nucleophile (halide). The electrophile attacks the double bond, stealing a pair of pi electrons and attaching them to the resultant molecule (carbocation). The nucleophile (halide) completes the reaction, resulting in the formation of a new molecule.
The following are some points that to conclude electromeric effect:
- The electromeric effect is only transient since it lasts only as long as the attacking reagent is present.
- The electromeric effect can be depicted using curved arrows, which indicate electron shift.
- There are two types of electromeric effects: positive electromeric effects and negative electromeric effects.
- When the attacking reagent is removed, the electromeric effect disappears.
Also read: Important Topic of Chemistry: Alkanes – Nomenclature
FAQs
What types of electrons are present in an electromeric effect?
The electromeric effect is mediated by pairs of pi electrons. The electromeric effect refers to the quick creation of a dipole in an organic compound molecule as a result of the full transfer of electron pi pairs shared by one of the atoms under the action of an intrusive reagent. This effect may be detected in molecules with at least one bond. When the atoms in this mass bond are exposed to an intrusive reagent, one pair of pi bonding electrons is totally transferred to one of the two atoms.
Why is an electromeric effect just temporary?
The electromeric effect is a transient effect that lasts only as long as the invasive reagent is present and expressed in the organic compound; once the invasive element is removed from the system, the polarised molecule recovers to its natural state. This effect is only seen in organic molecules with at least one single bond. The electromeric effect is characterized by the presence of pairs of electron pi and is not observed by ethers.
What is another name for the electromeric effect?
The electromeric effect is the loss of molecule polarizability caused by the removal of an intramolecular electron. As a result, it is also known as a 'conjugative mechanism' or, more generally, a 'tautomeric mechanism,' as it is defined by a single electron exchange into another octet of electron atoms.









