Table of Contents
Introduction
We comprehend and apply what we learn in real life by blending application-based learning of each topic in Science, which is a fascinating subject. That makes it more engaging for students and provides for a more comprehensive grasp of our surroundings as a whole. Chemistry is a branch of science that investigates substances, their chemical properties, and their interactions in various settings. Every atom contains a nucleus in the core that is made up of protons and neutrons.
Electrons spin along a certain axis around this nucleus. These electrons are arranged in shells that encircle the nucleus. The nucleus is in the heart of an onion, whereas the layers are the shells. Furthermore, the shells are stacked, much like an onion. And each shell contains a unique number of electrons that orbit the nucleus. As a result, an orbital configuration is a multilayer shell structure in which a specific number of electrons orbit around the nucleus. The electron configuration of an element describes how electrons are distributed in its atomic orbitals.
Overview
A molecule’s electronic configuration refers to the distribution of electrons in distinct molecular orbitals. Understanding the molecule is crucial. The number of electrons in a molecule’s or molecular ion’s bonding and antibonding molecular orbitals can be determined from its electronic configuration. An atom’s stability can be predicted based on electron configuration. Every shell contains a predetermined number of electrons. It is determined by a simple formula, where the maximum number of electrons for the nth shell is 2n2.
When all of an atom’s orbitals are occupied, it becomes the most stable and thus unreactive. Furthermore, the most stable electron configuration has a complete state of energy. Because these orbital configurations are important properties of noble gases, they do not easily react with other molecules. When two or more atoms come into touch with each other, the valence shells or outermost electrons of an atom interact first. When the valence shell of an atom is filled, it becomes the most stable. In general, the chemical behavior of an element is determined by its valence electrons. Elements with the same valence electrons, for example, have similar chemical characteristics.
Electron mobility is more sophisticated than it appears. They always take the same paths, which are known as orbitals or subshells. These orbitals come in a variety of forms, including s, p, d, and f. In that situation, the first energy level only has one s orbital, while the second has one s and three p orbitals. Similarly, the third energy level has three p orbitals and five d orbitals. S has the lowest energy of any orbital.
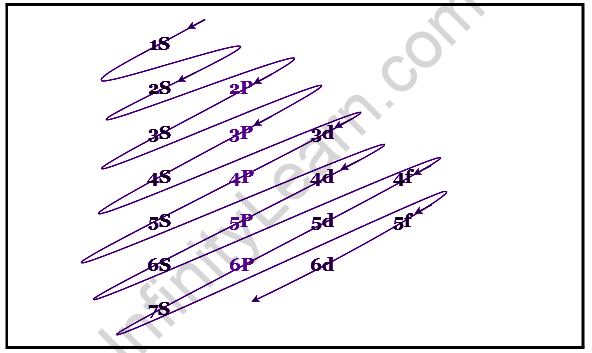
Electronic Configuration
Electron configurations are a description of where electrons are in relation to a nucleus. As previously stated, each neutral atom has the same number of electrons as protons.
The Aufbau principle determines the arrangement in which electrons are inserted into orbitals based on their energy. The lowest energy orbitals are the first to fill.
Chemists use electron configurations to explain and communicate the arrangement of electrons around an atom’s nucleus. This notation aids in forecasting how atoms will link together and form chemical bonds, as well as their behavior.
Electronic Configuration of an Element
Scientists place all electron-containing atomic subshells in a sequence (with the number of electrons they store written in superscript) to define how electrons are dispersed in an element’s atomic orbitals, following a standard nomenclature.
One element’s physical and chemical features can be linked to its unique electron configuration. The valence electrons, or electrons in the outermost shell, are responsible for the element’s distinct chemistry.
Electronic Configuration of an Atom
Generally, an atom’s electron configuration is a representation of how electrons are dispersed throughout the orbital shells and subshells.
The electron configuration is frequently used to define an atom’s orbitals in its ground state, but it may also be used to depict an atom that has ionized into a cation or anion by compensating for electron loss or gain in succeeding orbitals.
While allocating an atom’s electrons to orbitals, one must first understand the fundamental ideas of electron configurations. Electrons, with a negative charge, occupy electron orbitals, which contain 95 percent of the space in which they can be found, and these elements make up atoms which in turn make up the elements of the periodic table. The four types of orbitals (s,p,d, and f) have various forms, and each orbital can only house two electrons. Because the sublevels of the p, d, and f orbitals differ, they can carry more electrons.
It is critical to use the periodic table to establish the electron configurations of atoms, but it is also important to remember that there are certain principles to follow when allocating electrons to different orbitals. A periodic table is a fantastic tool for writing electron configurations.
Electronic Configuration of Copper
Different atomic orbitals arrange an element’s atoms’ electrons symbolically, forming its electronic configuration.
We know that the atomic number of copper = is 29
We can write the electronic configuration of copper.
Cu29= 1 s2,2 s2,2p6,3 s2,3p6,3 d10,4 s1
(Since fully filled orbitals are more stable, so one electron from 4 s orbital transfers into 3 d orbitals.)
Electronic Configuration of Chromium
Scientists use a standard nomenclature to sequence electron-containing atomic subshells (with the number of electrons they store written in superscript) and define how electrons are dispersed in an element’s atomic orbitals.
We know that, the atomic number of chromium = 24
We can write the electronic configuration of chromium.
1s22s22p63s23p43d54s1
As we know, half-filled and fully filled subshells have got extra stability.
So, one of the 4s2 electrons jumps to the 3d5 so that it is half-filled.
Also read: Stability Of Half-Filled And Completely-Filled Orbitals
FAQs
Q. An atom of fluorine contains nine electrons. Tell how many of these electrons are in s orbitals.
Ans: As the atomic number of fluorine is 9, the electronic configuration for fluorine is 1s22s22p5.
What role does the electronic configuration of elements play?
Elements’ electronic configuration is important in defining their chemical characteristics. Despite their small size, electrons have a significant impact on the nature of elements. They determine the element’s chemical properties, including valency, ionization potential, ionization enthalpy, chemical bonding, and nearly every other feature. When an element lacks an electron, it becomes an electron acceptor, and when it has an extra electron, it becomes an electron giver.
Q. Write the correct electronic structure for Mg2+.
Ans: The +2 charge on Mg2+ means that the molecule sheds two valence electrons to achieve a more stable orbital. Those electrons will be shed from the outermost valence shell, which in this case is the 3s shell; therefore, 1s22s22p6 is the correct electronic structure for Mg2+.









