Table of Contents
Introduction
Stability Of Half-Filled And Completely-Filled Orbitals: Inside any given atom, electrons move in an orderly arrangement of orbitals around the nucleus, with the attraction between electrons and nucleus overcoming repulsion among the electrons, which would otherwise cause them to fly apart. These orbitals are organized in concentric shells that radiate from the nucleus and have an increasing number of subshells. The electrons in the orbitals closest to the nucleus are held the most tightly, while those in the outermost orbitals are shielded by intervening electrons and are held the least tightly by the nucleus. Since electrons move around within this structure, they create a diffuse cloud of negative charge that fills nearly the entire volume of the atom.
Overview
An atom’s electron configuration portrays how electrons are distributed among the orbital shells and subshells. The electron configuration is commonly used to describe an atom’s orbitals in its ground state. Still, it can also represent an atom that has ionized into a cation or anion by compensating for electron loss or gain in subsequent orbitals. Many of an element’s physical and chemical properties can be linked to its unique electron configuration. The valence electrons, or electrons in the outermost shell, are responsible for the element’s distinct properties.
Because of their spin, each orbital can only have two electrons. An electron can have either a clockwise or counterclockwise spin about its axis, transforming it into a tiny magnet. Electrons in full orbitals are paired with spins or magnetic polarities that are opposite.
Stability of orbitals
As per Hund’s rule, atoms with half-filled or completely-filled orbitals are comparably more stable, so removing an electron from such atoms requires more energy. As a result, the ionization potential or ionization enthalpy of such an atom is considerably higher than expected based on its location in the periodic table.
The exceptional stability of half-filled and fully-filled electron configurations can be described using symmetry and exchange energy. The half-filled and totally-filled electron structures have symmetrical electron distributions, which leads to stability. Furthermore, electrons can exchange places in such a structure to the greatest extent possible. This interchange results in stabilization.
Stability of Half-filled Orbitals
The stability of exactly half-filled orbitals in degenerate orbitals is stronger than that of other partially filled configurations. This can be explained using symmetry and the concept of exchange energy. Half and completely-filled subshells become more stable because of the symmetrical distribution of electrons. Additionally, the exchange energy release is greatest in half-filled or filled subshells, boosting their stability. This is proportional to the number of exchanges of positive electrons by electrons with the same spins from one orbital to another in the same subshell.
It can be seen that in chromium, the electronic configuration is [Ar]3 d54 s1. Here, the 3rd orbital is half-filled, and there are ten possible exchanges, as shown in the figure.
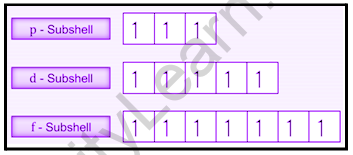
Hund’s rule, which enables maximal multiplicity, is based on the exchange energy, which states that electron pairing is possible only when all degenerate orbitals contain one electron each.
Stability Of Half-filled And Completely-filled Orbitals or Subshells
For writing electronic configuration, almost all elements follow the same pattern. When the energies of two subshells differ, an electron from the lower energy subshell may travel to the higher energy subshell.
This is due to two factors:
(1) Symmetrical distribution: As we all know, symmetry leads to stability. Because of the symmetrical distribution of electrons, orbitals in which the sub-shell is exactly half-filled or filled are more stable.
(2) Exchange energy: Those electrons in degenerate orbitals have a parallel spin and tend to exchange positions. The energy released during this process is referred to as exchange energy. The frequency of exchanges is most significant when the orbitals are half-full or filled. As a result, its stability is at its peak.
Two or more electrons with the same spin can swap places with the degenerate orbitals. The spinning of electrons creates a new quantum mechanical interaction known as Exchange energy. Additionally, the exchange energy release is greatest in half-filled or filled subshells, boosting their stability. This is proportional to the number of exchanges of positive electrons by electrons with the same spins from one orbital to another in the same subshell.
Even while electrons in the same subshell have the same energy, their spatial distribution is different, so their shielding effect on one another is rather minor. As a result, electrons are more drawn to the nucleus. And the state with the least total electronic energy always corresponds to an element’s ground state electronic configuration.
FAQs
Which electron configuration is the most stable?
It has been discovered that noble gases have the most stable orbital arrangement. The reason for this is that their valence shell is full. In the case of helium, the two valence electrons are found in the 1s sublevel, while the remaining eight electrons are found in the s and p subshells.
How are electrons arranged in an orbital?
An atom's nucleus is surrounded by orbitals, and electrons are organized on these orbitals. The electrons nearest to the nucleus, on the other hand, have the least energy, while the ones farthest away have the most. Furthermore, electrons generally migrate within an atom's orbitals or subshells.
How many electrons are there in a shell?
Every shell contains a predetermined number of electrons. It is determined by a simple formula, where the maximum number of electrons for the nth shell is 2n2. For example, the first shell may carry a maximum of two electrons, whereas the second and third shells can hold up to eight and eighteen electrons. The information about the stability of orbitals from various physics-related articles is available here. The stability of half-filled and completely-filled orbitals is an important topic in physics. Students who want to flourish in chemistry need to be well known about this to get deep knowledge about it to do well on their exams. The concepts and brief explanations are provided here to assist students in effectively understanding the respective topic. Continue to visit our website for additional chemistry help.








