Table of Contents
Solubility product:
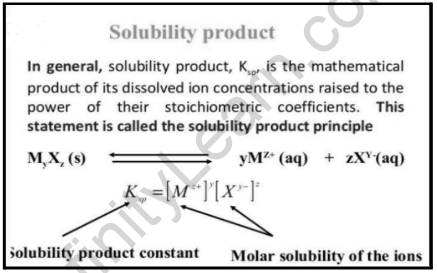
The equilibrium constant for a solid that breaks in an aqueous solution is the solubility product constant (Ksp). All of the rules for calculating equilibrium constants remain in effect. Once a reaction has reached equilibrium, an equilibrium constant is the ratio of the concentration of the products divided by the concentration of the reactants. The lower the solubility, the smaller the solubility product. Solubility products can help you forecast whether or not a precipitate will develop under certain conditions. The change (x) in the equilibrium calculation equals the solid’s solubility.
A brief outline
The solubility product constant:
To characterize saturated solutions of ionic substances with low solubility, solubility product constants are utilized. The dissolved, dissociated ionic component and the undissolved solid are in a condition of dynamic equilibrium in a saturated solution. The equilibrium constant is known as the solubility product constant because it is the product of the concentrations of the ions present in a saturated solution of an ionic molecule. Its symbol is Ksp. Calculate and apply solubility product constants in a range of applications.
- Calculating Ksp’s from data on solubility
- Calculating an ionic compound’s solubility in pure water using its Ksp
- Calculating an ionic compound’s solubility in a solution containing a common ion
- When two solutions are joined, it is determined if a precipitate will develop or not.
Important concepts
Assume you have barium sulfate and its saturated aqueous solution. The equilibrium between undissolved solids and ions is represented by the following equation:
BaSO4 (saturated solution in water) ⇌ Ba2+(aq) +SO−4(aq)
The equilibrium constant in the overhead case is:
K = [Ba2+] [SO−4] [BaSO4]
In the situation of pure solid substances, the concentration remains constant, and so we could tell:
Ksp = K[BaSO4] = [Ba2+] [SO−4]
The solubility product constant (Ksp) is what it’s called. This also means that when solid barium sulfate is in equilibrium with its saturated solution, the product of barium and sulfate ion concentrations equals the solubility product constant.
The Importance of Solubility Product solubility is determined by a variety of factors:
- The most important of which is the salt’s lattice enthalpy and the solvation enthalpy of the ions in the solution.
- When a salt is dissolved in a solvent, the interactions between ions and the solvent must overcome the strong forces of attraction of the solute (lattice enthalpy of its ions).
- Ions have a negative solvation enthalpy, which indicates that energy is released throughout the process.
- The amount of energy liberated during solvation, referred to as solvation enthalpy, is determined by the nature of the solvent.
- The solvation enthalpy of non-polar solvents is low, indicating that this energy is insufficient to overcome the lattice enthalpy.
- As a result, non-polar solvents do not dissolve the salts.
- As a result, the solvation enthalpy of salt must be greater than its lattice enthalpy in order for it to dissolve in a solvent. Temperature affects solubility, which is different for each salt.
pH has an impact on solubility.
The solubility of a solute can be affected by the pH of an aqueous solution. You can modify the charge state of the solute by changing the pH of the solution. When the pH of a solution is such that a specific molecule has no net electrical charges, the solute seems to have little solubility and precipitates out of the solution. The isoelectric point, or pI, is the pH where the net charge is neutral.
The significance of a solubility product is as follows:
1)The magnitude of a substance’s solubility product determines its solubility.
2)The solubility product is a heterogeneous equilibrium constant, or a special form of the equilibrium constant.
3) It’s important in saturated fluids where an ionic component hasn’t dissolved completely. Because the temperature of solubility products varies, the temperature at that they were evaluated must always be given.
Significance of solubility product in NEET exam
The NEET forums are used to reveal and respond to the most commonly asked questions on the test. These can be described in essential terms with the use of notes from experienced analysts in the field, which are available on the Infinity Learn online stage. Different choice inquiries are obvious to practice, assuming that pupils deal with an increased enthusiasm for the courses in general throughout the instructive program.
Reactions are given by subject-matter experts and gifted teachers. The reactions follow CBSE and NCERT guidelines for the NEET exam, assisting students in attaining better scores. The courses are fairly rated, and there are a few free courses available for prospective students to try out.
Also read: Heat Capacity and Specific Heat
FAQs
What is the difference between a solubility product constant and a solubility constant?
The entire amount of a chemical that can be dissolved in a solvent at equilibrium is referred to as solubility. The solubility product constant, across the other hand, is an equilibrium constant that sends details on the equilibrium between solid solute and its fragmented constituent ions across the solution.
Q. What governs the value of Ksp?
Ans: Below is some critical aspects that govern the solubility product constant:
- The impact of the common ion
- The ion-diversity phenomenon
- There are ion-pairs that exist.
Q. What then is the calcium chloride solubility product constant?
Ans: Calcium chloride has the molecular formula CaCl2. One calcium chloride molecule separates into one calcium cation & two chloride anions when dissolved in polar liquids. The following is a representation of the equilibrium reaction.
CaCl2 ⇌ Ca2+ + 2Cl–
As a result, the solubility product constant could be described as follows:
Ksp = [Ca2+] [Cl–]2
Q. What is the formula for magnesium fluoride’s solubility product constant?
Ans: One magnesium fluoride molecule disintegrates into one magnesium cation and two fluoride anions when dissolved in polar liquids. Below is a depiction of the equilibrium reaction.
MgF2 ⇌ Mg2+ + 2F–
Consequently, the solubility product constant could be stated as:
Ksp = [Mg2+] [F–]2