Table of Contents
Introduction
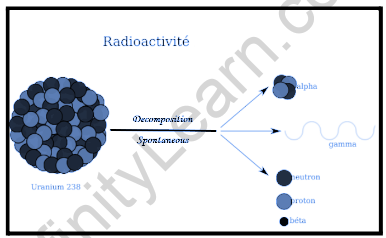
The ability of certain forms of matter to emit energy and subatomic particles spontaneously is known as radioactivity. It’s essentially a property of specific atomic nuclei. An unstable nucleus will spontaneously dissolve, or decay, into a more stable structure, but only in a few precise ways, for example by generating specified particles or electromagnetic energy. In addition, the daughter nucleus may be able to release any extra energy in the form of penetrating electromagnetic radiation. With the flow of time, the number of remaining parent nuclei, as well as the amount of radiation emitted, declines.
Radioactivity is a physical entity, not a biological one. Simply put, the radioactivity of a sample could be determined by counting the number of atoms that decay spontaneously each second. Instruments designed to detect the specific type of radiation released for each “decay” or disintegration can be used to do this. It’s possible that the exact figure of disintegrations per second is rather high.Anatomic nucleus’ destabilization does not prompt it to emit radiation straight away. Instead, the chance of an atom deteriorating is constant, as if unstable nuclei are always playing a lottery, with random drawings to choose which atom will emit radiation and dissolve towards a more stable state. The half-life is indeed the length of time it takes for half of an atom in a given mass to “win the jackpot,” that is, emit radiation and shift to a more stable state. The half-lives of various atoms range from about a second to billions of years.
A brief outline
Radioactivity is the process of emitting radiation spontaneously, as the term implies. This is accomplished by an unstable atomic nucleus that “wants” to give up some energy in order to switch to a more stable configuration. A Sample of modern physics was expanded in understanding why this occurred throughout the first part of the twentieth century, follow-on in nuclear decay being fairly well acknowledged by 1960. When a nucleus has too numerous neutrons, it releases a negative beta particle, which alters one of the neutrons to a proton.
When a nucleus has too many protons, it emits a positron (positively charged electron), which converts a proton to a neutron. A nucleus with too much energy will generate a gamma-ray, which will waste a lot of energy without affecting any of the particles in the nucleus. A nucleus with too much mass emits an alpha particle, expelling four hefty particles (two protons and two neutrons).
Important concepts
Alpha rays:
Two protons and two neutrons bonded together form a particle corresponding to a helium-4 nucleus that makes up alpha rays or alpha radiation. The alpha particle is represented by the symbol α or α2+. They are also written as He2+ or 24He2+, denoting a helium ion with a +2 charge, because they are similar to helium nuclei (missing its two electrons). The alpha particle becomes a regular (electrically neutral) helium atom 42He after gaining electrons from its environment.
The net spin of alpha particles is zero. Alpha particles have a kinetic energy of about 5 MeV and a velocity of roughly 4% of the speed of light due to the process of their generation in typical alpha radioactive decay. (For the limits of these figures in alpha decay, see the discussion below.) They are a highly ionizing kind of particle radiation with a shallow penetration depth (when arising from radioactive alpha decay) (stopped by a few centimeters of air, or by the skin). Long-range alpha particles produced by ternary fission, on the other hand, are three times more energetic and reach three times as far.
Beta particles:
A beta particle, also known as a beta ray or beta radiation (β), is a high-energy, fast-moving electron or positron produced by the radioactive disintegration of an atomic nucleus. Beta-decay can be divided into two types: decay and + decay, which create electrons and positrons, respectively. In the air, beta particles with an energy of 0.5 MeV have a range of around one meter; the distance varies with particle energy. Beta particles are a form of ionizing radiation that is more ionizing than gamma rays but less ionizing than alpha particles for radiation protection reasons. The stronger the ionizing effect, the more damage to living tissue occurs, yet the lower the radiation’s penetrating capability.
Beta particles are utilized as tracers and can be used to treat diseases such as eye and bone cancer. The most frequent substance utilized to make beta particles is strontium-90. Beta particles are also employed in quality control to determine the thickness of an item passing through a roller system, such as paper. While flowing through the product, some beta radiation is absorbed. If the product is produced too thick or too thin, it will absorb a variable amount of radiation. The rollers are then moved by computer software that monitors the quality of the created paper in order to modify the width of the final result.
Gamma rays:
A gamma-ray, commonly known as gamma radiation (), is a penetrating kind of electromagnetic radiation that results from atomic nuclei decaying radioactively. It is constituted of electromagnetic waves with the shortest wavelengths, often less than X-rays. Radioactive decay and secondary radiation from air interactions with cosmic ray particles are the most common natural sources of gamma rays that originated on Earth. Other uncommon natural sources of gamma rays, such as terrestrial gamma-ray flashes, create gamma rays as a result of electron activity on the nucleus. Fission, such as that which occurs in nuclear reactors, and high-energy physics experiments, such as neutral pion disintegration and nuclear fusion, are two notable artificial sources of gamma rays.
Uses of gamma rays:
Aside from the use of nuclear energy to generate electricity, radioactivity has several applications in medical physics, earth sciences, industry, and cultural heritage preservation.
These are the properties that are used in these diverse applications:
- Radioactivity decreases over time.
- Emission of radiation
- Detection sensitivity
Radiotherapy and/or ionizing radiation treatment are the most common uses of radiation in medicine. It became obvious a few months after the discovery of X-rays, over a century later, that biological action radiation may be used to cure tumors. Cancer cells divide faster and are more vulnerable to ionizing radiation than normal ones. It is feasible to kill these cells and eradicate the tumor by giving them a certain amount of radiation.
Significance of isotones in NEET exam
Infinity Learn is here to help you achieve your full potential by providing you with the best instructors available for each topic. The solutions have been developed in accordance with the most recent NEET exam. The materials are intended to assist students in revising the entire chapter and performing well on tests. NEET solutions are based on the most recent exam criteria and norms. It gives students a solid understanding of the chapter as well as straightforward explanations of the exercise problems, with a focus on key ideas. Its purpose is to assist pupils in resolving their doubts.
Also read: Important Topic Of Physics: Radioactive Decay Law
FAQs (Frequently asked questions)
In Gamma Decay, what is emitted?
During gamma decay, the nucleus generates photons, which are little chunks of electromagnetic energy with a shorter wavelength. The number of protons and neutrons in the parent nucleus and daughter nuclei remains constant during gamma decay.
What Are the Applications of Radioactive Particles?
Radioactive particles are commonly utilized in medicine, particularly for therapeutic and diagnostic goals. Radioactive materials are utilized in biomedical research to test new medications and study bone development and changing cellular processes in mammals. Additionally, radioactive elements can be employed for industrial purposes such as identifying new energy sources, protecting blood and basic food supplies, warning fires, and improving town road and highway safety.
How far and how far can radioactivity radiation travel?
The travel distance of radioactive particles, as well as their penetration power, is determined by the type of radiation. The alpha and beta radiation particles are unable to travel long distances and can be easily prevented. Gamma rays, on the other hand, are extremely difficult to stop due to their long wavelength. Neutrons and x-rays may also travel a long distance and are commonly utilized in industrial and academic settings. Only dense hydrogen-packed materials can prevent penetrating radiation.









