Table of Contents
Introduction:
A cell has two terminals, one positive and one negative. The positive terminal is referred to as the cathode, while the negative terminal is referred to as the anode. They are both electrodes in a cell. Two or more cells are linked serially or parallelly to form a battery. A wire connects the cell’s terminal to form a closed circuit. Electric current flows through the wire from the positive terminal of the cell to the negative terminal, while positive ions in the electrolyte flow from lower to higher potential, providing some resistance to the current flow.
Internal Resistance of a Cell
The electromotive force, or EMF, is a concept that most students are unfamiliar with. These concepts are inextricably linked to the more familiar concept of voltage. Understanding the difference between these two and what EMF means, in general, provides us with the tools we need to solve many problems in physics and electronics. It will also introduce the concept of a battery’s internal resistance. Again, we can say that EMF tells us about the voltage of the battery without taking into account the Internal Resistance, which reduces the value.
This topic explains the emf formula using examples, allowing us to learn the Internal Resistance Formula again, a mathematical equation used to calculate the resistance of a moving object.
Note:
If you are preparing for Engineering Entrance Exams such as JEE Main and BITSAT, it is critical that you prepare this Physics topic on the Internal Resistance of a Cell. Read this article to learn about the Internal Resistance of a Cell and the related formula for internal resistance of a cell, among other things! Also, review relevant diagrams and FAQs related to the Internal resistance of the cell to get a sense of the types of questions that will be asked in the exam.
Overview of Internal Resistance of a Cell
Various circuit elements maintain continuity when the electric current in an external circuit flows from the cell’s positive terminal to its negative terminal, and the electrolyte of the cell provides resistance to current flow, which is referred to as the cell’s internal resistance.
Also read: Important Topic of Physics: Kirchhoff’s Laws
It is the resistance (opposing force) in the flow of current caused by the electrolyte and electrodes in the battery/cell. Also, it is found in the cell or battery and it is measured in ohms.
Consider the following circuit. The cell can be modified by connecting an emf ‘E’ and an internal resistor with resistance r in series. A resistance R external load resistor is also connected across the circuit. The terminal potential difference, denoted by V, is the potential difference between the cell’s positive and negative terminals when current flows through the circuit.
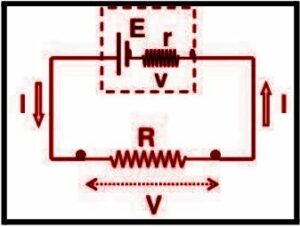
Understand the Internal Resistance of an electric motor or any other electrical device and how to reduce it in order to improve efficiency. Electrical engineers utilize Internal Resistance as a key concept for many projects and experiments related to electricity, as well as improving engine performance and fuel efficiency in Internal Combustion Engines (ICE).
Electrochemical Cell
An electrochemical cell is a device that may create electrical energy from chemical reactions or use electrical energy to speed up chemical reactions. These devices can convert chemical energy into electrical energy and vice versa. A typical 1.5-volt electrochemical cell powers electrical appliances such as TV remotes and clocks. Galvanic and Voltaic cells generate electric current through chemical reactions, while electrolytic cells create chemical reactions when an electric current is applied to them.
The diagram below depicts the various components of an electrochemical cell.
Example and Diagram
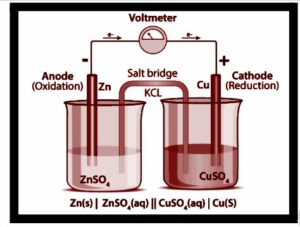
A salt bridge links the two half cells of an electrochemical cell, providing a platform for ionic contact while preventing them from mixing. Oxidation occurs in one half cell, while the other half cell undergoes reduction. It results in an equilibrium reaction and a net voltage of 0. To measure the electrode potentials, a standard hydrogen electrode is used as a reference electrode.
Electromotive Force
An inductor can generate an electromotive force (EMF) that opposes the incoming power, resulting in a negative EMF. An electrochemical cell can convert energy from one form to another using a generator to produce electromotive forces (EMF). Faraday’s law is also implemented in the electromagnetic flowmeter.
Electromotive Force of a Cell
The Nernst equation can be used to calculate the electromotive force (EMF). It is also known as the maximum potential difference between two electrodes of a cell. This EMF determines if an electrochemical cell is galvanic and can convert chemical energy into electrical energy. The specific type of cell determines its voltage. Although temperature differences and other factors can cause deviations from this ideal value.
FAQ’s
1. How do you calculate a cell’s internal resistance?
Ans: A graph that is said to be of a terminal that is the p.d. versus current.
The intercept on the y-axis is said to be equal to the cell’s e.m.f. in this case. Gradient of the graph is said to be equal to -r, where r denotes the Internal Resistance of the cell.
2. What are the causes of internal resistance?
Ans: We can say that sulfation and grid corrosion, which are the main causes of the rise in Internal Resistance, will result in acid. The temperature, which also affects heat resistance, lowers it while cold raises it. We can say that heating the battery temporarily lowers the Internal Resistance, allowing for more runtime.
3. What are some examples of common applications?
Ans: Understanding the Internal Resistance of batteries, practicing Internal Resistance Formulas on simple projects or tasks, and applying Internal Resistance when studying batteries or engines in cars or trucks are all examples of how the Internal Resistance Formula can be used.






