Table of Contents
Generally, enthalpy and internal energy change are measured using an experimental technique known as calorimetry. Such techniques are based on thermometric procedures performed in a calorimeter vessel submerged in a known liquid volume. The heat produced during the procedure is evaluated by quantifying the temperature difference using known heat capacities of the calorimeter and liquid. Particularly, enthalpy and internal energy can be measured under two distinct conditions. This included constant pressure, which is referred to as enthalpy, and constant volume, which is referred to as internal energy. Enthalpy is the amount of heat energy that is evolved or absorbed during the course of a chemical reaction. It is represented by the letter H, and the letter H indicates the amount of energy. The change in enthalpy is given by ∆H, where the delta symbol represents the change and the unit is joules or kilojoules.
Overview
We can say that thermodynamics is the branch of science concerned with the relationship between heat and other forms of energy, such as work. It is frequently summarized as three laws that describe the constraints on how different types of energy can be interconverted. Chemical thermodynamics is the branch of thermodynamics concerned with chemical reactions. Energy can be transferred between systems and their surroundings in a variety of ways. The thermodynamic terms enthalpy and internal energy are used to explain this energy exchange.
Enthalpy is defined as the sum of the system’s internal energies. The reason for this is that a change in internal energy occurs during a chemical reaction, and this change is calculated as enthalpy. The addition of a system’s potential and kinetic energy is referred to as its internal energy. The stored energy is known as potential energy, and the energy released as a result of molecule movement is known as kinetic energy. Furthermore, the internal energy is denoted by U, and change in internal energy is denoted by ∆U.
Internal energy and enthalpy are the same for a given system at constant pressure. Internal energy shifts can occur in two ways: due to heat and due to work.
Calorimetry
Calorimetry is a technique for measuring the amount of heat involved in a chemical or physical process. Calorimetry is a technique for calculating the amount of heat transferred to or from a substance. To accomplish this, heat is exchanged with a calibrated object (calorimeter). The amount of heat is converted from the change in temperature of the calorimeter’s measuring part (since the previous calibration was used to establish its heat capacity).
Calorimetry Analysis
This method of measuring heat transfer necessitates the definition of a system (the substance or substances undergoing the chemical or physical change) and its surroundings (the other components of the measurement apparatus that serve to either provide heat to the system or absorb heat from the system). Knowing the heat capacity of the surroundings, as well as taking careful measurements of the masses of the system and surroundings, as well as their temperatures before and after the process, enables one to calculate the heat transferred as described in this section.
A calorimeter is a device that measures the amount of heat in a chemical or physical process. When an exothermic reaction occurs in solution in a calorimeter, for example, the heat produced by the reaction is absorbed by the solution, raising its temperature. When an endothermic reaction occurs, the heat required is absorbed from the solution’s thermal energy, lowering its temperature.
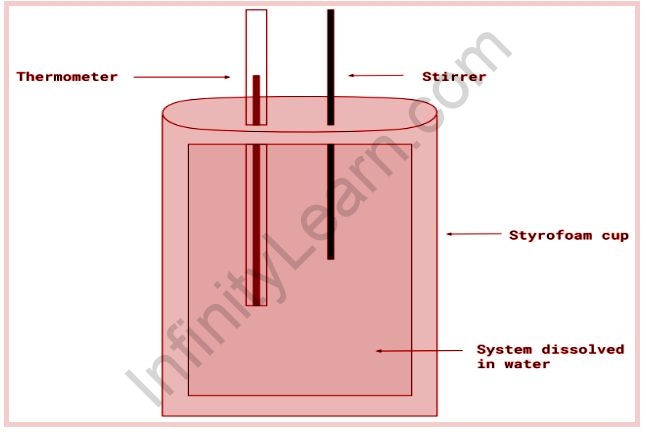
A calorimeter is a set of nested foam cups fitted with a lid to limit the heat exchange between the liquid in the cup and the air in the surrounding environment. In a typical calorimetry experiment, specific amounts of the reactants are dispensed into separate containers, and the temperatures of each are recorded. They are then combined in the calorimeter, where the reaction begins. The reactant mixture is constantly stirred until the reaction is complete, and the temperature of the reaction is constantly monitored.
Use of Calorimeter
Calorimeters are devices that measure the volume and heat produced over a specific time period. That is, calorimeter is a device used to calculate the enthalpy, or heat energy, of a reaction. Calorimeters of varying quality are available. In high school chemistry classes, simple calorimeters are used, but in industry, much more sophisticated calorimeters are used.
Measurement of ∆U and ∆H
Measurement of enthalpy change(∆H):
The energy released at constant pressure is defined as enthalpy. It is the sum of internal energy plus the product of pressure and volume.
H=U+PV
Here,
H= enthalpy
U= internal energy
P= pressure of the system
V= volume of the system
It is hard to calculate a system’s absolute enthalpy directly. As a result, we compute the enthalpy about a reference point. As a result, we typically measure the change in enthalpy. Endothermic reactions have a positive enthalpy change, while exothermic reactions have a negative enthalpy change. Calorimetry techniques are used in the laboratory to measure enthalpy change.
We already know that enthalpy change is the heat change at constant pressure, which is
∆H=qp. The term “coffee-cup calorimeter” is frequently used to calculate enthalpy change. In this technique, the cup is partially filled with a known volume of water, and a thermometer is inserted through the cup’s lid so that its bulb is below the water’s surface. The heat of a chemical reaction is absorbed by water when it occurs. The change in temperature of the water is used to calculate the amount of heat absorbed or evolved. Because the cup is made of polystyrene foam, which is an excellent insulator, very little heat energy escapes.
In this process, the energy change or enthalpy change is calculated as:
ΔH=qp=mcpΔT
Here,
m is said to be the mass of water
cp is said to be the specific heat capacity of water at constant pressure
ΔT is said to be the temperature difference
Measurement of change in internal energy ∆U
The energy change at constant volume is referred to as internal energy change. A bomb calorimeter is typically used to measure internal energy change. In this technique, a steel vessel (commonly known as a bomb) is immersed in a water bath to ensure that no heat is lost to the surrounding environment. In the oxygen gas supplied by the bomb, a combustible substance is burned. The heat emitted is absorbed by the water surrounding the bomb, and the temperature change is measured. Because volume does not change in the completely sealed bomb calorimeter, energy changes associated with the reaction are measured at constant volume. Because the volume remains constant, the system’s work done is nil. The process’s energy change, or internal energy change, is calculated as follows:
ΔU=qv=mcvΔT
Here,
m= mass of water
CV= specific heat capacity at constant volume
ΔT= temperature difference
Also read: Heat Capacity and Specific Heat
Frequently Asked Questions
What is the working principle of a calorimeter?
The calorimetric working principle states that in an insulated system, the heat energy lost by the hot body equals the heat energy gained by the cold body.
Why Copper is used in the calorimeter?
Cu is a good heat conductor. Because Cu has a low SHC, it quickly reaches equilibrium temperature by absorbing a small amount of heat. This ensures that the calorimeter absorbs or releases a small amount of heat during the heat exchange process.
How does a coffee cup calorimeter work?
The cup is partially filled with a defined volume of water, and a sensitive thermometer is inserted through the cup's lid, with its bulb below the water's surface. When a chemical reaction occurs in the coffee cup calorimeter, the water absorbs the heat produced by the reaction.









