Table of Contents
Regarding the properties of closed systems in thermodynamic equilibrium, the third law of thermodynamics states: When a system’s temperature approaches absolute zero, its entropy approaches a constant value. This constant value cannot be affected by any of the other parameters that define the closed system, such as pressure or the applied magnetic field. At absolute zero (zero kelvins), the system must be in the lowest possible energy state. Entropy is proportional to the number of accessible microstates, and there is usually one unique state (referred to as the ground state) with the lowest energy. The entropy at absolute zero will be exactly zero in this case.
If the system does not have a well-defined order (for example, if the order is glassy), there may be some finite entropy remaining as the system is brought to very low temperatures, either because the system becomes locked into a configuration with non-minimal energy or because the minimum energy state is non-unique. The constant value is referred to as the system’s residual entropy. Entropy is essentially a state-function, which means that the inherent value of various atoms, molecules, and other particle configurations, including subatomic or atomic material, is defined by entropy, which can be discovered near 0 K. The third law of thermodynamics’ Nernst–Simon statement is concerned with thermodynamic processes at a fixed, low temperature: As the temperature at which the process is performed approaches 0 K, the entropy change associated with any condensed system undergoing a reversible isothermal process approaches zero. A condensed system refers to liquids and solids in this context. It is impossible for any process, no matter how idealized, to reduce a system’s entropy to its absolute-zero value in a finite number of processes, according to Nernst’s famous formulation (which is actually a result of the Third Law). A formulation of the third law exists that approaches the subject by postulating a specific energy behavior: If the composite of two thermodynamic systems constitutes an isolated system, then any energy exchange in any form between those two systems is bounded.
Overview
According to the third law of thermodynamics, the entropy of a perfect crystal at zero Kelvin (absolute zero) is equal to zero. Entropy, represented by the letter ‘S,’ is a measure of disorder/randomness in a closed system. It is proportional to the number of microstates (a fixed microscopic state that a system can occupy) accessible to the system; that is, the greater the number of microstates a closed system can occupy, the greater its entropy. The ground state of the system is the microstate in which the energy of the system is at its lowest. In a closed system at zero Kelvin, the following phenomena can be observed: There is no heat in the system. The system’s atoms and molecules are all at their lowest energy levels. As a result, at absolute zero, a system has only one accessible microstate – its ground state. The entropy of such a system is exactly zero, according to the third law of thermodynamics.
The entropy of flawless crystals at (OK) temperature is defined by the third law of thermodynamics. It has a significant impact on how modern industries operate and the principles upon which our machines are designed. These rules apply to the entire universe. These laws, which are stated in reference to some standards, explain physical conditions in terms of thermodynamics. The third law of thermodynamics, in particular, discusses the universe as a whole and the magnitude of randomness within it. This law basically treats everything as a single system and establishes rules for the randomness of the system’s components in terms of their temperature dependence. The third law of thermodynamics states that as a system’s temperature approaches absolute zero, its entropy approaches a constant value. This implies that when the temperature reaches absolute zero, there is a minimum measure of randomness in a system. When two thermodynamic systems are combined to form an isolated system, any energy exchange in any form between those two systems is bounded. This is also explained by the fact that at absolute zero, the system contains no heat, indicating the presence of all molecules at their lowest energy points.
Third thermodynamics law
According to the third law of thermodynamics, the entropy of a system at absolute zero is a well-defined constant. This is because a system at zero temperature exists in its ground state, so its entropy is determined solely by the ground state’s degeneracy. The entropy of a perfect crystal is zero when its temperature is equal to absolute zero, according to the third law of thermodynamics (0 kelvin).
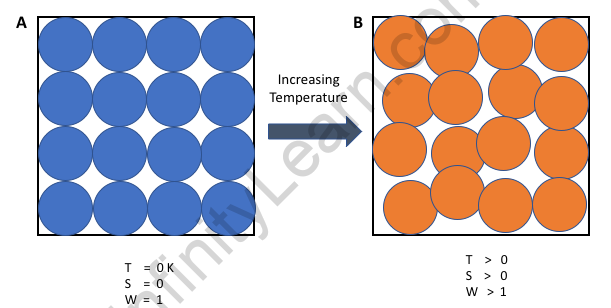
Application of third law of thermodynamics
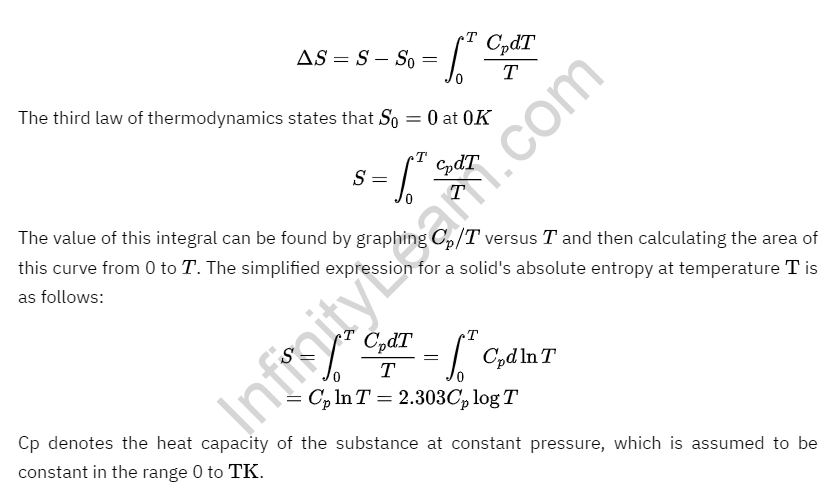
The third law of thermodynamics has an important application in calculating the absolute entropy of a substance at any temperature ‘T.’ The heat capacity measurements of the substance are used to make these determinations. If S0 is the entropy at 0 K and S is the entropy at T K for any solid, then
 Also read: Second Law of Thermodynamic
Also read: Second Law of Thermodynamic
FAQs
Give an example of the Third Law of Thermodynamics.
Water vapours are the gaseous forms of water at high temperatures and are an example of the third law of thermodynamics. The molecules in the steam move at random. As a result, it has a high Entropy. If these vapours are set to cool this steam to less than 100 degrees Celsius, it will be transformed into water, where the movement of the molecules will be limited, resulting in a decrease in Entropy.
What role does the Third Law of Thermodynamics play in refrigeration?
The third law states that no environmental parameter can be changed to cause a change in the entropy of a system at absolute zero. As a result, refrigeration is a series of alternating steps. The first is to maintain a constant temperature while changing other parameters to extract heat from a system, resulting in a decrease in entropy. The next step is to insulate the system and reverse the parameter change. As a result, the temperature drops. So, in general, alternate isothermal and adiabatic processes work on the third law principle and aid in better refrigeration.



