Table of Contents
Several theories have been offered to explain the nature of coordination compound bonding. The Valence Bond Theory was created to use quantum physics to explain chemical bonding. This theory is primarily concerned with the formation of individual bonds from the atomic orbitals of the atoms involved in the formation of a molecule. Crystal Field Theory is one of the most widely accepted theories for explaining coordination complex bonding. Hans Bethe came up with the idea. It treated atoms as hard spheres, and their interaction is purely electrostatic.
The central metal atom is positively charged, while the ligands surrounding it are negatively charged. When this negatively charged ion approaches the positively charged ion, electrostatic attraction causes changes in the metal ion’s energy levels and, eventually, bonds to form. Crystal Field Theory was able to explain the bonding in the majority of complexes. It did, however, have some limitations. The limitations of the Crystal field theory will be discussed in this article.
Crystal field theory is a model for describing the electronic structure of transition metal compounds, which are all coordination complexes. CFT accounts for some magnetic properties, colours, hydration enthalpies, and spinel structures of transition metal complexes, but it does not attempt to describe bonding. CFT was invented in the 1930s by physicists Hans Bethe and John Hasbrouck van Vleck. Later, CFT was combined with molecular orbital theory to create the more realistic and complex ligand field theory (LFT), which sheds light on the chemical bonding process in transition metal complexes. Valence bond theory is abbreviated as VBT.
It is a theory that describes how different chemical bonds between atoms form. This theory explains how atomic orbitals overlap or mix to form chemical bonds.
CFT is an abbreviation for Crystal field theory. It is a model that is intended to explain the breaking of degeneracies (equal energy electron shells) of electron orbitals (typically d or f orbitals) caused by a static electric field created by a nearby anion or anions (or ligands). The primary distinction between VBT and CFT is that VBT explains orbital mixing while CFT explains orbital splitting.
Overview
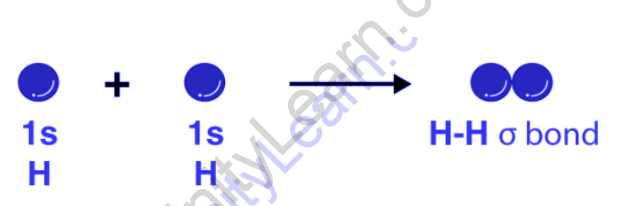
The Lewis approach to chemical bonding fails to provide insight into the creation of chemical bonds. Furthermore, only a few applications of the valence shell electron pair repulsion theory (or VSEPR theory) were known (and also failed in predicting the geometry corresponding to complex molecules). To solve these challenges, German physicists Walter Heinrich Heitler and Fritz Wolfgang London created the valence bond hypothesis. The Schrodinger wave equation was also used to explain how two hydrogen atoms created a covalent connection. The chemical bonding of two hydrogen atoms is depicted below using the valence bond theory.
The ideas of electronic configuration, atomic orbitals (and their overlapping), and atomic orbital hybridization are all included in this theory. Chemical bonds are produced by atomic orbitals overlapping and electrons being concentrated in the bond area. The electrical structure of molecules created by the overlapping of atomic orbitals is also explained by the valence bond theory. It also emphasises how one atom’s nucleus is drawn to the electrons of the other atoms in a molecule.
Coordination complex bonding is explained by Crystal Field Theory. In this theory, atoms are considered as hard spheres, and their interactions are solely electrostatic. The ligands surrounding the core metal atom are negatively charged, while the central metal atom is positively charged. Negatively charged ions and neutral molecules are among the ligands that surround the core metal atom. As a result, they attract the positive charge of the centre atom through both electrostatic and covalent attraction. Relying entirely on electrostatics, this complicates comprehending their bonding. Understanding how metal-ligand connections develop necessitates knowledge of both electrostatics and covalent interactions. When a negatively charged ion approaches a positively charged ion, electrostatic attraction produces changes in the metal ion’s energy levels, and the metal ion finally bonds to the positively charged ion.
Crystal Field Theory Versus Valence Bond Theory
Crystal field theory satisfactorily explains several of the features of complexes that could not be addressed by valence bond theory. CFT is, therefore, unquestionably superior to vbt, as evidenced by the following advantages of cft over vbt: Instead of the dramatic change predicted by VBT, CFT predicts a gradual change in magnetic characteristics of complexes. Simple temperature changes may impact the magnetic characteristics of certain compounds when Δ is extremely close to P. In contrast to VBT, which can only forecast or explain magnetic behaviour beyond specifying the number of unpaired electrons, the CFT provides a theoretical basis for understanding and predicting fluctuations of magnetic moments with temperature as well as precise magnetic properties of complexes. Though the assumptions that VBT and CFT are based on are drastically different, the fundamental difference is in how they describe the orbitals that are not occupied in low spin states. VBT prohibits their usage since they are involved in the formation of hybrid orbitals, whereas CFT strongly discourages their use because they are repelled by the ligands. The link between the metal and the ligand is covalent according to VBT, but merely ionic according to CFT. The bond is currently seen as having both ionic and covalent properties. In contrast to valence bond theory, CFT provides a framework for quickly interpreting tetragonal distortions, for example. The interaction between a metal ion and a ligand is caused by the attraction between the metal ion’s positive charge and the ligand’s unpaired electrons (negative charge). This theory is primarily based on the changes that occur in five degenerated electron orbitals (a metal atom has five d orbitals). When a ligand approaches a metal ion, the unpaired electrons are closer to some d orbitals than to other d orbitals of the metal ion. This leads to a loss of degeneracy. The electrons in the d orbitals repel the ligand’s electrons (both are negative charged). As a result, d orbitals closer to the ligand have higher energy than other d orbitals.
Limitations of Crystal Field Theory
Assumptions are always at the heart of theories. These assumptions may be perfectly consistent with experimental data. However, CFT has some limitations, which are discussed below:
- Only d orbitals are considered in this theory. The contributions of s and p blocks were never considered.
- It regarded atoms as hard spheres, with electrostatic interactions between them. However, it cannot be true in reality.
- The orbitals of the ligands were not given any weight in this theory. It only mentioned the central metal ion
- This theory was unable to explain the behaviour of some complexes, as well as why some orbitals split significantly more than others.
- It does not explain why H2O is a stronger ligand while OH- is weaker.
- One of the major disadvantages of CFT is that it does not account for the covalent character of metals and ligands. It only took into account the ionic character.
- Crystal Field Theory does not explain all of the consequences and effects that exist as a result of the covalent character.
Limitations of Valence Bond Theory
The valence bond theory has several flaws.
- Failure to account for carbon’s tetravalency.
- There is no information provided about the energies of the electrons.
- The theory assumes that electrons are concentrated in certain areas.
- It does not provide a quantitative interpretation of coordination compounds’ thermodynamic or kinetic stabilities.
- There is no differentiation between weak and strong ligands.
- The colour of coordination compounds has no explanation.
FAQ’s
What exactly is valence bond theory?
It is a theory that explains chemical bonding. According to VBT, the overlap of partially filled atomic orbitals results in the formation of a chemical bond between two atoms. The unpaired electrons are shared, resulting in the formation of a hybrid orbital.
What are the drawbacks of VBT?
The valence bond theory fails to explain carbon's tetravalency and also fails to provide insight into the energies associated with electrons. The theory also assumes that electrons are concentrated in specific areas.
How do the limitations of Crystal Field Theory explain coordination complex bonding?
The Crystal Field Theory's limitations account for the covalent interaction of the metal and ligand atoms. Because the theory does not account for these interactions, it leaves a gap in our understanding of coordination complexes. This is where Ligand Field Theory steps in to fill the gap by emphasising the covalent nature of bonding in coordination complexes. Because of the covalent nature of bonding, coordination complexes are formed by sharing electrons. This is why some ligands are stronger than others while others are weaker.



