Table of Contents
Introduction
Bronsted-Lowry theory is also known as the proton theory of acids and bases. In 1923, the theory was presented independently by Danish chemist Johannes Nicolaus Bronsted and by English chemist Thomas Martin Lowry that any compound that can transfer a proton to any other compound is described as acid and the proton-receiving compound. proton is defined as the base. Proton is considered a radio particle with a positive electric charge unit, in which the H + signal can be represented as it forms the nucleus of a hydrogen atom.
About Theory
The material acts as an acid only when there is a base, according to the Bronsted Lowry system; similarly, an object only acts as a base where there is acid. In addition, when an acidic substance is devoid of the proton, it forms a base called the acid conjugate base, and when a photon is detected with a basic substance, it produces an acid called base conjugate acid. Therefore, the reaction between an acidic substance, such as hydrochloric acid, and a basic substance, such as ammonia, can be represented by the equation given below:
HCl +NH3⇌NH4++Cl–
In the ratio given above, ammonium ion (NH+4) is given as an acid conjugate to base ammonia, and ion chloride (Cl–) is given as a conjugate base for hydrochloric acid.
Bronsted-Lowry theory also increases the number of compounds, which are considered acids and bases so that they include not only neutral molecules (for example, alkali metal hydroxides and nitric, sulfuric, and acetic acid) but also other molecules and acetic acid. atoms have both positive and negative energy costs (called cations and anions). Hydronium ions, ammonium ions, and other hydrated metals are considered acids. Phosphate, acetate, sulfide, carbonate, and halogen ions are considered the basis.
Definitions of Acids and Foundations
According to Arrhenius’s theory, acids are defined as solvents in an aqueous solution to form H+ (hydrogen ions), while bases are defined as solvents in an aqueous solution to form OH– (hydroxide ions).
Naturalist chemists Thomas Martin Lowry in England and Johannes Nicolaus Bronsted in Denmark both proposed the doctrine in 1923 with their own names. Acids and foundations are represented in the theory of Bronsted Lowry in the way they share, which makes it very common. This definition is expressed in terms of equity.
Acid + base ⇌ conjugate acid + conjugate base.
With HA acid, this equation can be illustrated as follows:
HA + B ⇌ A– + HB+
A rating symbol (⇌) can be used because reactions are possible in both forward and reverse directions. HA acid can lose protein to become its conjugate base, A–. The base, B, can receive a proton to convert to conjugate acid, HB+. Most acid-base reactions are rapid so the reaction components often have a dynamic balance with the others.
Aqueous Solutions
Consider the reaction below the acid-base:
CH3COOH + H2O⇌CH3COO– + H3O+
Acetic acid, CH3COOH, is acidic due to the fact that it contributes to the protein in water (H2O) and becomes its base of the conjugate, which is acetate ion (CH3COO−). H2O is given as a base because it receives a proton from CH3COOH and converts it into its conjugate acid, which is a hydronium ion (H3O+).
The acid-base reverse reaction is also an acid-base reaction between the acid conjugate base and the base conjugate acid in the initial reaction. In the example given above, acetate is given as the basis for reverse reactions, and hydronium ion is given as an acid.
H3O++ CH3COO–⇌CH3COOH + H2O
The strength of the Bronsted-Lowry theory is that it does not need acid to separate, in contrast to Arrhenius’ theory.
Comparison with the Lewis Acid-Base Theory
In the year when Bronsted and Lowry published their theory, G. N. Lewis proposed a different/alternative theory of acid-base reaction. Lewis’ theory is based on the structure of electronics. The Lewis base can be described as a compound, which can donate an electron pair to Lewis acid, which is a compound that can receive an electron pair. Lewis’s proposal describes the division of Bronsted – Lowry electronically.
HA + B⇌A– + BH+
Both the base of the conjugate, A-, nucleus, B, appears in the image above holding a single pair of electrons, a proton, which is Lewis acid which can be transferred between them.
Bronsted-Lowry (Proton acid and base) theory is an acid-base reaction theory, introduced by Johannes Nicolaus Bronsted (Danish Chemist) and Thomas Martin Lowry (English Chemist) in 1923. In theory, the acid and the base react with each other and by the exchange of proton acid, it forms its own conjugate base and the base forms its combined acid.
Bronsted-Lowry theory is an extended version of Arrhenius’ acid-base theory.
FAQ’s
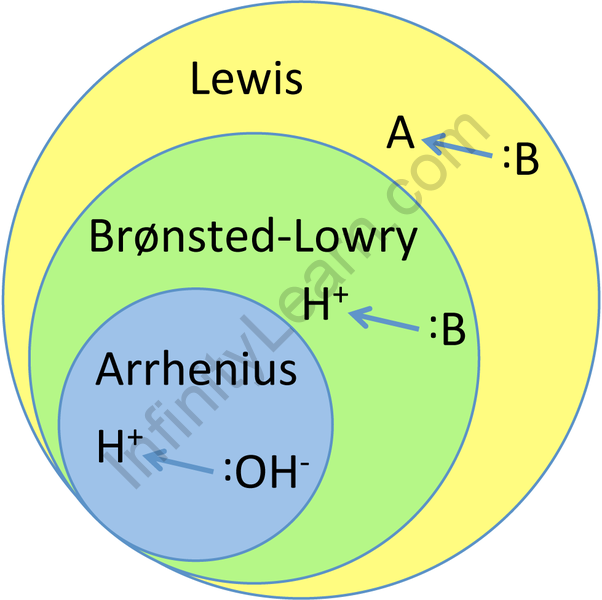
Q. Give a comparison between Bronsted Lowry, Arrhenius, and Lewis acids and bases?
Ans: Arrhenius’s theory is a protonic concept. Any substance that emits H + ions when dissolved in water is called acid. HCl is an example. And the factor that separates it from producing OH- ions when dissolved in water is called the base. NaOH is an example.
Bronsted Lowry’s theory is the concept of accepting proton suppliers. The main source of protons is acid. HCl is an example. Also, the proton receiver is called the base, of which Cl- is the model.
Lewis’s theory is an electronic concept. A pair of donating electrons is called the base — for example, all the nucleophiles. Also, the receiver of an electron pair is an acid-for example, all electrophiles.
Q. Write down three types of acid-base theory?
Ans: Let’s take a look at acid-base ideas.
Arrhenius’s theory of acids and bases – Acids are a substance that produces hydrogen ion in solution, and foundations are substances that produce hydroxide ion in solution.
Bronsted Lowry Theory of Acids and Bases – Acids are provided as a proton supplier, and the base is provided as a proton receptor.
Lewis’s theory of acid and bases – Acid is an electron pair receiver, and the base is an electron-pair supplier.





