Table of Contents
Hybridization Of BeCl2: Beryllium chloride is an associated chemical compound with the formula BeCl2. it’s a colourless, absorptive solid that dissolves well in several polar solvents. Its properties are just like those of aluminium chloride, because of beryllium’s diagonal relationship with aluminium.
STRUCTURE AND SYNTHESIS OF BERYLLIUM CHLORIDE
Beryllium chloride is ready by reaction of the metal with atomic number 17 at high temperatures
Be + Cl2 → BeCl2
BeCl2 can even be ready by carbothermal reduction of metallic element compound within the presence of atomic number 17. BeCl2 may be ready by treating metallic elements with acid.
Two forms (polymorphs) of BeCl2 are far-famed. each structure consists of tetrahedral Be2+ centres interconnected by doubly bridging chloride ligands. One kind comprises edge-sharing polytetrahedra. the opposite kind resembles metallic element halide with interconnected adamantane-like cages. In distinction, BeF2 may be a three-d compound, with a structure corresponding to that of quartz.
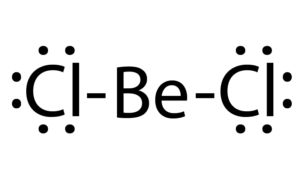
In the gas part, BeCl2 exists each as a linear chemical compound and a bridged compound with 2 bridging atomic numbers 17 atoms wherever the metallic element atom is 3-coordinate.[5] The linear form of the monomeric kind is as expected by VSEPR theory. The linear form contrasts with the monomeric varieties of a number of the dihalides of the heavier members of a cluster a pair of, e.g. CaF2, SrF2, BaF2, SrCl2, BaCl2, BaBr2, and BaI2, that are all non-linear. metallic element chloride dissolves to present tetrahedral [Be(OH2)4]2+ particle in binary compound solutions as confirmed by undulation spectroscopic analysis.
HYBRIDIZATION
In chemistry, orbital cross (or hybridization) is the conception of blending atomic orbitals to make new hybrid orbitals (with totally different energies, shapes, etc., then the element atomic orbitals) appropriate for the pairing of electrons to make chemical bonds in valence bond theory. for instance, in a very atom that forms four single bonds the valence-shell s orbital combines with 3 valence-shell p orbitals to make four equivalent sp3 mixtures in a very tetrahedral arrangement around the carbon to bond to four totally different atoms. Hybrid orbitals are helpful within the clarification of molecular pure mathematics and atomic bonding properties and are symmetrically disposed of in-house. typically hybrid orbitals are shaped by intermixture atomic orbitals of comparable energies.
HYBRIDIZATION OF BeCl2
To know concerning the pairing of BeCl2 (Beryllium Dichloride) we’ve to require a more in-depth scrutinize the central atom that is Be. Its electronic configuration is 1s2, 2s2, wherever 2 electrons are gifted within the valence shell. throughout the formation of BeCl2, metallic element atom bonds with 2 Cl atoms via single valence bonds. the number of negatron pairs around the central atom is 2. No lone combine is found within the molecule. If we have a tendency to analyze this info then we will conclude that BeCl2 has sp pairing.
TIPS FOR HYBRIDIZATION OF BeCl2
In the BeCl2 molecule, metallic element bichloride forms single valency bonds with 2 chemical element atoms every.
The central atom Be can comprise 2 bond pairs.
No lone try exists.
FAQs
What is the hybrid state and form of BeCl2?
In BeCl2, the hybrid state is sp, because the electron of atomic number 4 is a pair of and there area unit a pair of chemical element atoms, therefore no lone try gift. therefore the hybrid state is sp. there's no amendment of hybrid state in {phase amendment|phase transition|state change|physical change|natural process|natural action|action|activity} however there's a change of structure, or we will say orientation.
Is BeCl2 linear?
The shape of the BeCl2 molecule is linear.



