Table of Contents
Introduction
Individual molecules in gases have all of the physical attributes of an element, including mass, momentum, and energy. The density of a gas is calculated by dividing the total mass of all molecules by the volume of the gas. The pressure of the gas is used to calculate the linear momentum of the gas. When the molecules of gas collide with the walls of the container in which they are contained, the molecules give momentum to the walls, which results in a force that can be measured. The temperature of a gas is a measurement of the gas’s average kinetic energy. The gas molecules are constantly moving and producing energy as a result of this movement. The motion is directly proportional to the temperature. These are some key themes taught in the kinetic theory of gases section of the IIT JEE syllabus.
Assumptions in Gas Kinetic Theory
Some assumptions underpin the kinetic theory of gases. Let’s take a look at some of these assumptions.
- The molecules or atoms of gases are always in random motion and never remain still.
- The gas’s molecules or atoms collide with one other and with the walls of the container in which the gas is housed on a regular basis.
- Gas particles are exceedingly minute, and when compared to the overall capacity of the container, the total volume filled by these particles is deemed inconsequential.
- Between the particles of the gases, there is a minimal force of attraction.
- The average kinetic energy of gas particles is proportional to the gas’s real temperature, therefore all gases with the same temperature have the same kinetic energy.
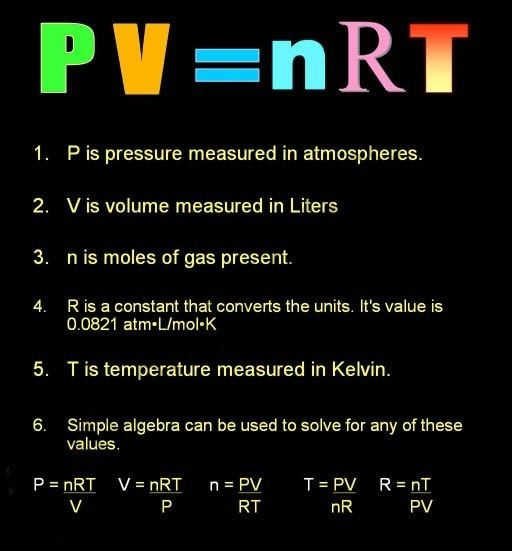
Kinetic Theory of Gas Formulas
Boltzmann’s Constant
kB = nR/N
The Boltzmann constant is kB.
The gas constant is R.
The number of moles is denoted by the letter n.
The number of particles in a mole is N. (the Avogadro number)
Total Translational K.E of Gas
K.E = (3/2)nRT
The number of moles is denoted by the letter n.
The universal gas constant is R.
The absolute temperature is denoted by T.
Maxwell’s Law of Distribution
Vrms > V> Vp
Vrms is the RMS speed.
The average speed is denoted by the letter V.
The most likely speed is Vp.
Internal Energy
U = (f/2) nRT
For n moles of an ideal gas.
FAQs
What exactly is RMS?
RMS (Root Mean Square) is the abbreviation for Root Mean Square. In an AC system, the root means square voltage or current is also known as time-averaged voltage or current. A statistical measure of the size of a changing quantity is the root mean square, which can also be called a quadratic mean. It's most effective when the function's values alternate between negative and positive. The square root of the arithmetic mean of the squares of the original values is the RMS value of a collection of variables.
What is the Boltzmann Constant, and what does it mean?
The Boltzmann Constant was invented by Max Plank. Ludwig Boltzmann was the inspiration for the name. It is a physical constant that is calculated by dividing the ratio of two constants known as the gas constant and the Avogadro number. The Boltzmann constant is utilized in a variety of physics areas, including describing the equipartition of an atom's energy and the Boltzmann factor. In the statistical concept of entropy, this constant is very important.
What is the kinetic theory of gases formula?
For molecules with f degrees of freedom, K = (f/2) KвT. Boltzmann's constant is KB. The temperature of the gas is denoted by T. For an ideal gas with n moles.