Table of Contents
Halogens are really the elements in group 17. Fluorine, chlorine, bromine, iodine, and astatine were indeed halogen elements. Such elements are too reactive to occur naturally, but their compounds are global. Chlorides are by far the most abundant; fluorides, bromides, and iodides are less common but still readily available.
Halides with less electronegative elements are formed by halogens. In this, metal halides range from ionic to covalent, whereas nonmetal halides are covalent. Interhalogens are being formed by combining two or more different halogens. Minerals have been directly reacted with all of the representative halogen elements or with hydrohalic acid solutions (HF, HCl, HBr, and HI) to produce representative metal halides. In the addition of aqueous hydrohalic acids, basic anions such as hydroxides, oxides, or carbonates are involved.
Properties of the Halogens
- Chlorine may appear as a pale yellow-green gas (left), gaseous bromine as a deep orange gas (centre), and gaseous iodine as a purple gas (right). Fluorine seems to be extremely reactive and should be avoided at all costs.
- Bromine is really only slightly soluble in water, but it dissolves completely in less polar (or nonpolar) solvents such as chloroform, carbon tet, and compound, forming solutions that range from yellow to reddish-brown depending on concentration.
- Iodine would seem to be soluble in chloroform, carbon tet, compound, and a wide range of hydrocarbons, resulting in violet solutions of I2 molecules. It is also quite soluble in iodide aqueous solutions, with which it forms brown solutions. Because iodine molecules have empty valence d orbitals, they can act as weak Lewis acids towards the iodide ion, resulting in brown solutions.
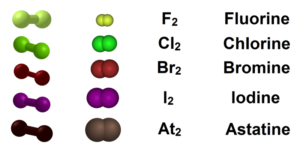
Halogens Characteristics
- Such elements are all extremely reactive.
- Because of their current high reactivity, halogens cannot exist as pure elements in the environment. They have been typically found as compounds or as ions.
- Many halogen ions and atoms have been frequently found in combination with other compounds found in seawater or drinking water. That might be because halogen elements tend to form salt after coming into contact with metals and combining with them to form compounds.
- As said before, halogens are the sole elemental group within the entire tabular array, which consists of elements belonging to one or more of the three classical states of matter (solid, liquid, and gas). It really is demonstrated by the fact that, when kept at room temperature and pressure, astatine and iodine form solids, bromine forms a liquid and chlorine and fluorine from gases.
- After combining with hydrogen to form binary compounds, all halogen elements form hydrogen halides, which are potent acids.
- The halogen group tends to react with itself to form diatomic interhalogen compounds.
- Halogens have a high tendency to react with other matter due to their atoms’ high levels of electronegativity, which could be due to the high sufficient nuclear charge of all halogen atoms.
FAQs
What are the general trends in halogen chemistry?
There may several patterns in halogen chemistry that do not require double or triple bonds to explain the chemistry of the halogens. Fluorine chemistry has been simplified by the fact that it is the first electronegative element in the tabular array and that it has no d orbitals in its valence shell, so it cannot expand its valence shell. Chlorine, bromine, and iodine all have valence shell d orbitals that can be extended to carry up to 14 valence electrons. The chemistry of the halogens has been conquered by oxidation-reduction reactions.
Are halogens gases?
alogens were indeed highly reactive nonmetal elements found in Periodic Table Group 17 of the periodic table. At room temperature, halogens seem to be solids, liquids, and gases that vary in colour.



