Table of Contents
Introduction:
In everyday life, we come across chemicals that are mixes of several elements and compounds. Homogeneous mixes are formed when components are combined together in such a way that their composition remains consistent throughout the sample. A heterogeneous mixture, on the other hand, is one that does not have a homogeneous composition across the sample.
Homogeneous mixes are also known as solutions, and solutions can comprise solids, liquids, and/or gases as constituents. We frequently wish to be able to quantify the amount of a species in a solution, which is referred to as the concentration of that species. In this post, we’ll look at how to quantitatively characterize solutions and how that information may be used when doing stoichiometric calculations.
Chemists use molarity to quantify the concentration of a solution, allowing them to compare concentrations while calculating chemical reactions and working with chemical solutions. A concentration is a term used by chemists to describe the quantity of material dissolved in a given amount of solution.
For one major reason, molarity is extremely significant in chemistry. It is a method of determining the concentration of any composition. The molarity of any solution is a method of determining the individual components or compounds present in any solution. Molarity is calculated by dividing the amount of a solute by the number of litters of its solutions. You may quickly determine the precise quantity of any element or compound in a solution using molarity computation.
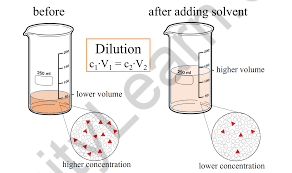
Concentration is a term used frequently in chemistry and related subjects. It is a measurement of how much one material is combined with another. This term can relate to any form of chemical combination, although it is most typically used in the context of solutions to refer to the quantity of solute dissolved in a solvent. More solute or less solvent must be added to concentrate a solution (for instance, by selective evaporation). To dilute a solution, either more solvent or less solute must be added. There is a concentration at which no more solute will dissolve in a solution. At this moment, we may claim that the solution is saturated. If the extra solute is added to a saturated solution, it will not dissolve. Instead, phase separation will occur, with either coexisting or suspended phases occurring. A multitude of parameters, including ambient temperature and the particular chemical composition of the solvent and solute, influence the point of saturation.
Molarity is an important notion in stoichiometric calculations involving reactions in solution, such as precipitation and neutralization.
Molar concentration (Molarity)
When it comes to the molar concentration of the solution, the solvent is the chemical component that makes up the greatest percentage of the solution. Solutes are any compounds that are combined with the solvent. Solutes can take the form of solids, liquids, or gases.
A mole is a unit of measurement for a chemical compound, from which the term “molarity” is derived. Molarity, commonly known as the molar concentration of a solution, is a technique for estimating the quantity of material contained in a certain chemical solution. It is calculated by taking two indications into account: the number of moles in the solute and the volume of the solution. The volume of the solution is mostly determined in litres. The letter ‘M’ stands for ‘Molarity.’
The solvent is the component of a solution that is present in the greatest amount. A solute is any chemical species that are mixed in the solvent, and solutes might be gases, liquids, or solids. The Earth’s atmosphere, for example, is composed of 78 per cent nitrogen gas, 21 per cent oxygen gas, and 1 percent argon, carbon dioxide, and other gases. Consider the atmosphere to be a solution in which nitrogen gas serves as the solvent and oxygen, argon, and carbon dioxide serves as the solutes.
A solute’s molarity, also known as its molar concentration, is defined as the number of moles of solute per litre of solution.
Molarity = No. of Moles of Solution / Volume of solution in Liters
Molarity is measured in mol/litre units.
If we use symbols to represent molarity, then
M = n/V
Where ‘M’ denotes molarity, ‘n’ the number of moles of solute present in the solution, and ‘V’ is the volume of solution contained in a container.
The molar concentration of a solute is commonly shortened by adding square brackets around the solute’s chemical formula. The concentration of chloride ions in a solution, for example, can be represented as [Cl–]. We may convert between the volume of the solution and the moles (or mass) of the solute using molar concentration.
FAQ’s
Why is the volume of a solid solute not taken into account when determining molarity?
To solve this question, you will need to use the molarity formula and your prior knowledge of the solution. You already know that when a solid is completely miscible in a solvent, the volume of the resulting solution is the same as that of the solute.
What's the distinction between molarity and molarity?
Molarity (M) is a way of estimating the concentration of a solute inside a solution, which is represented in liters when the solution is nothing more than a combination of solutes and solvents. It takes into account the volume of the solution. Molality(m) is a way of estimating the concentration of a solute inside a solvent, expressed in kilograms. It takes the mass of the solvent into account.
Does the concentration of a solution vary when the volume of the solution changes?
The answer is yes when it comes to molarity. Even if we consider concentration by mass, it will still fluctuate unless the material has an indeterminate density. Because a substance's mass varies with its volume, so does its concentration. However, if the volume/mass/moles of both the solute and the solvent grow or decrease in an equal ratio, the concentration and molarity stay unchanged.
How do you compute the number of moles?
To get the molar mass, use the molecular formula; divide the mass of the compound by the molar mass of the compound, measured in grammes, to get the number of moles.



