Table of Contents
Amines are one of the main classes of natural mixtures which can be derived when we supplant at least one hydrogen particle of alkali atoms with an alkyl group.
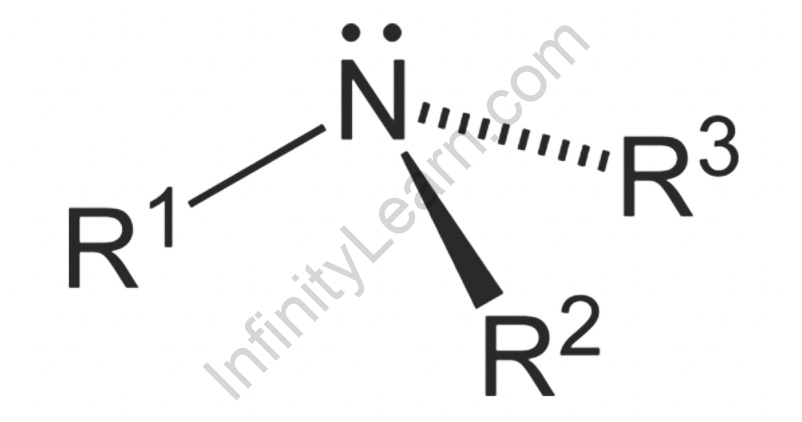
Amine Structure
- Nitrogen has 5 valence electrons as is trivalent with a solitary pair. According to VSEPR hypothesis, nitrogen present in amines is sp3 hybridized and because of the presence of solitary pair, it is pyramidal rather than tetrahedral shape which is an overall design for most sp3 hybridized atoms. Every one of the three sp3 hybridized orbitals of nitrogen cross-over with orbitals of hydrogen or carbon relying on the configuration of amines. Because of the presence of solitary pair, the C-N-H point in amines is under 109 degrees which is a trademark point of tetrahedral math. The point of amines is close to around 107 degrees.
Occurrence of Amines
- Naturally, amines happen in proteins, nutrients, chemicals, and so forth and they are additionally arranged synthetically to make polymers, medications, and colours.
Types of Amines
- Based on how the hydrogen iotas are supplanted by a smelling salts particle, amines can be separated into 4 kinds.
1. Primary Amines
- Whenever one of the hydrogen iotas of the smelling salts atom is supplanted by an alkyl or aryl group.
- Eg: Methylamine CH3NH2, Aniline C6H5NH2
2. Secondary Amines
- Two natural substituents supplant the hydrogen iotas of the smelling salts particle framing an amine.
- Eg: Dimethylamine (CH3)2NH, Diphenylamine (C6H5)2NH
3. Tertiary Amines
- At the point when each of the 3 of the hydrogen iotas are supplanted by a natural substituent, it very well may be an aryl or aromatic group.
- Eg: Trimethylamine N(CH3)3, Ethylenediaminetetraacetic acid (EDTA)
Cyclic Amines - These are auxiliary or tertiary amines in an aromatic ring structure. Eg: Piperidine (CH2)5NH, Aziridines C2H5N
Preparation of Amines
A few cycles for planning essential amines are referenced beneath.
Preparation of Primary Amines
1. Making of amines from halogenoalkanes
- This interaction will be completed in a fixed cylinder. Here haloalkanes will be warmed with the concentrated arrangement of alkali in ethanol. The blend can’t be warmed under the reflux as alkali would move out as gas from a holder.
- Presently coming to the arrangement of essential amine from halogenoalkane the reaction happens in two phases. Salt will be shaped at the main stage. Here ethyl ammonium bromide is the salt. It is like ammonium bromide with the exception of the way that one of the hydrogens in the ammonium molecule is supplanted by an ethyl group.
- An opposite reaction can happen between alkali and salt. It is delineated in the above reaction.
2. Reduction of nitriles
- We can get essential amines when nitriles are decreased with lithium aluminum hydride. This technique is principally utilized for the readiness of amines which contain one carbon particle more than the beginning amine.
3. Gabriel phthalimide union
- We can get essential amines effectively by Gabriel union. In this cycle, on the treatment of phthalimide with ethanolic potassium hydroxide, we get potassium salts of phthalimide. At the point when this is additionally warmed with alkyl halide followed by antacid hydrolysis then essential amine is delivered. We can’t plan aromatic essential amines because aryl halides don’t go through nucleophilic substitution with the anion which is framed by phthalimide.
Basicity of Amines
- Like alkali, essential and auxiliary amines have protic hydrogens and consequently they grandstand a level of acidity. Though tertiary amines have no protic hydrogen and along these lines don’t have a level of acidity.
- pKa an incentive for essential and auxiliary amines is around 38, which makes them a genuinely feeble acid. Though assuming we take the pKb, it is around 4. This makes the amines significantly more essential than acidic. Accordingly, a watery arrangement of an amine is emphatically soluble.
Uses of Amines
Amines have a broad application in our day to day routines. A few purposes of amines are recorded underneath:
- It is utilized in water cleaning, medication assembling and advancement of insect poisons and pesticides.
- It is engaged with the development of amino acids which is the structure square of proteins in living creatures. Numerous assortments of nutrients are likewise made by amines.
- Serotonin is a significant amine that capacities as one of the essential synapses. It controls the sensations of appetite and is basic for the speed with which the cerebrum works overall.
- Torment easing drugs, for example, Morphine and Demerol which are otherwise called analgesics are produced using amines.
From the above conversation, you would have acquired some comprehension of the fundamental ideas of amines however to find out about amines, chemical and physical properties of amines.
FAQs
What is the order for basicity of Amines?
Due to the presence of solitary pair electrons on the nitrogen iota of amine, it can give a couple of electrons to shape a base. The basicity of amines relies on the following elements, steric factors, Electronic element and dissolvable components, In the vaporous express the request for basicity of amine, when R=Me, 30 > 20 > 10. In the fluid arrangement the request for basicity of amine, when R= Me, 20 > 10 > 30 In the fluid arrangement the request for basicity of amine, when R= Et, 20 > 30 > 10
How many types of amine are there?
Contingent on the quantity of alkyl or aryl groups connected to the nitrogen particle, the aliphatic or aromatic amines are named essential, auxiliary and tertiary amines.







