Table of Contents
Introduction
The equilibrium constant of a substance response (typically indicated by the image K) gives knowledge into the connection between the items and reactants when a synthetic response arrives at harmony. For instance, the balance steady of fixation (signified by Kc) of a synthetic response at balance can be characterized as the proportion of the centralization of items to the convergence of the reactants, each raised to their particular stoichiometric coefficients. It is vital to take note that there are a few unique sorts of balance constants that give connections between the items and the reactants of balance responses as far as various units.
For a compound response, the harmony steady can be characterized as the proportion between how much reactant and how much item is utilized to decide substance conduct.
At harmony, Rate of the forward response = Rate of the regressive response
for example i.e. rf = rb Or, kf × α × [A]a[B]b = kb × α × [C]c [D]d
At a specific temperature, the rate constants are steady. The proportion of the rate steady of forwarding response to the rate consistent of in reverse response should be steady and is called a harmony consistent (Kequ).
Harmony Constant Formula
Kequ = kf/kb = [C]c [D]d/[A]a [B]b = Kc
where Kc, shows the harmony consistent estimated in moles per litre.
For responses including gases: The harmony steady recipe, as far as incomplete strain will be:
Kequ = kf/kb = [[pC]c [pD]d]/[[pA]a [pB]b] = Kp
Where Kp shows the harmony consistent equation as far as incomplete tensions.
Bigger Kc/Kp values demonstrate higher item arrangement and higher rate transformation.
Lower Kc/Kp values demonstrate lower item arrangement and lower rate transformation.
Medium Kc/Kp values show ideal item development.
Units of Equilibrium Constant
Harmony is consistent in the proportion of the fixations raised to the stoichiometric coefficients. Thusly, the unit of the balance steady = [Mole L-1]△n.
where, ∆n = amount of stoichiometric coefficients of items – the number of stoichiometric coefficients of reactants.
Balance Constant, Reaction Quotient and Gibbs Free Energy
K is the proportion of the general measure of items to reactants at balance while Q is the proportion any time of the season of the response. The Q worth can measure up to K to decide the course of the response to happen. The immediacy of the cycle is connected with the free energy change. △G (Gibbs Free Energy), K (Equilibrium Constant), and Q (Reaction Quotient) are connected as follows:
- △G < 0 and Qc ˂ Kc or Kp toward the beginning of the response: The response will continue to shape items.
- △G = 0 and Qc = Kc or Kp at harmony and no more changes in the grouping of the blend.
- △G > 0 and Qc > Kc or Kp after harmony: The response will continue toward the path to shape reactants.
Kc = Equilibrium steady estimated in moles per litre.
Kp = Equilibrium consistent determined from the fractional tensions
Connection among kc and kp
Think about the accompanying reversible response: cC + dD ⇒ aA + bB
The balance is consistent for the response communicated as far as the fixation (mole/litre):
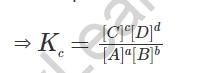
In the event that the balance includes vaporous species, the fixations are supplanted by incomplete tensions of the vaporous substances. The balance steady as far as fractional tensions is:
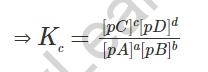
Where pA, pB, pC and pD addresses the halfway tensions of the substance A, B, C and D separately. In the event that gases are thought to be great, as indicated by ideal gas condition:
pV = nRT or p = nRT/V
Where,
- p is the tension in Pa,
- V is the volume in m3,
- n is the number of moles of gas,
- n/V = molar focus = [C]
- T is the temperature in Kelvin,
In the event that C is in mol dm3 and p is in bar, R = 0.0831 bar dm3 mol-1 K-1 or p = CRT
Filling in for pressure, as far as focus:
Dad = [A] RT; pB = [B] RT; pC = [C] RT and pD = [D] RT
Subbing these qualities in articulation for Kp:
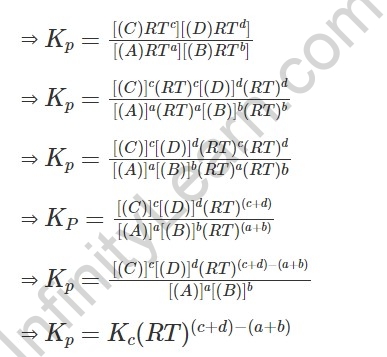
Where, △n = (c+d) – (a+b) for example number of moles of vaporous items – number of moles of vaporous reactants in the decent substance response.
Attributes of Equilibrium Constant
- It is response explicit and at a consistent temperature, it is fixed.
- An impetus changes the pace of forward and in reverse responses similarly not to influence the worth of the balance consistent.
- Changes in focus, pressure, temperature, idle gases might influence the harmony, inclining toward one or the other forward or in reverse response yet not the balance steady.
- Is connected with the standard free energy as, △G0 = -RT ln Kequ.
- For a similar reversible response, Kequ has various qualities at various temperatures.
- The harmony consistent of the opposite balance is proportional to the first balance for example Krev = 1/Kequ.
- Assuming the balance response stoichiometry is changed, the force of the harmony steady likewise gets changed by a similar amount.
- On the off chance that the balance is steady for the response, A + B ⇌ C + D is K, then, at that point, for harmony response 3A = 3B ⇒ 3C + 3D is K3
- On the off chance that stepwise different equilibria prompt the end results, the balance consists of the net balance = result of each stepwise harmony constant. Subsequently, the net harmony steady K = K1 × K2 × K3.
- Concurrent harmony responses, having a typical item. The balance steady of the responses don’t change. Because of the greater convergence of the normal item, the item fixations will be decreased.
Utilizations of Equilibrium Constant
Balance Constant For Predicting the Extent of Reaction
The balance consistent (Kc) can be utilized to anticipate the degree of a response, for example, the level of the vanishing of the reactants. The greatness of the balance steady gives a thought of the overall measure of the reactants and the items.
Case 1: The bigger worth of the balance steady (>103) shows that forward response is inclined toward for example the convergence of items is a lot bigger than that of the reactants at balance.
For Example:
- H2(g) + Br2(g)⇌ 2HBr(g) ⇒ Kc = 5.4×1018
- H2(g) + Cl2(g)⇌ 2HCl(g) ⇒ Kc = 4×1031
- H2(g) + 12O2(g) ⇌ H2O(g) ⇒ Kc = 2.4×1047
This shows that in harmony, a grouping of the items is extremely high, for example, response go nearly to the end.
Case 2: Intermediate worth of harmony consistent (10-3 to 103) show that the convergence of the reactants and items are similar.
For Example:
- Fe3 (aq) + SCN (aq) ⇌ [Fe(SCN)]2 (aq) ⇒ Kc = 138 at 298 K
- H2 (g) + I2 (g) ⇌ 2HI (g) ⇒ Kc = 57 at 700 K.
Case 3: Low worth of harmony consistent (<10-3) shows that retrogressive response is leaned toward for example grouping of reactants is a lot bigger than that of items for example the response continues to a tiny degree in the forward bearing.
For Example:
- N2 (g) + O2 (g) ⇌ 2NO (g) ⇒ Kc =4.8 × 10-31 at 298K
- H2O (g) ⇌ H2 (g) + (1/2) O2 (g) ⇒ Kc = 4.1 × 10-48
Balance Constant for Predicting the Direction of a Reaction
The balance consistent can be utilized to foresee the bearing of the response. We really want a term, response remainder (Qc communicated as far as focuses or Qp as far as halfway tensions) like the balance consistent aside from that the circumstances are not at harmony.
For a fair response, aA + bB ⇌ cC + dD
Response remainder (Qc or Qp) is given as:
Qc = [C]c[D]d/[A]a[B]b
Qp = pcC × pdD / paA × pbB
Correlation with Kc and the heading of Reaction:
- On the off chance that Q = Kc , the response is in balance [Where, Kc = harmony constant]
- On the off chance that Q > Kc, Q will more often than not decline in order to become equivalent to K. Subsequently, the response will continue in the retrogressive bearing.
- On the off chance that Q < Kc, Q will quite often increment to become equivalent to K. Thus, the response will continue in the forward heading.
FAQs
How would you track down the equilibrium constant?
Compose the balance articulation for the response. Decide the molar focuses or halfway tensions of every species included. Decide all balance focuses or incomplete tensions utilizing an ICE outline. Substitute into the harmony articulation and tackle for K.
What does the balance steady K 1 show?
Assuming that the worth of K is more prominent than 1, the items in the response are inclined toward. In the event that the worth of K is under 1, the reactants in the response are inclined toward. On the off chance that K is equivalent to 1, neither reactants nor items are leaned toward.
What are Kc and Kp ?
Kc = Equilibrium steady estimated in moles per litre. Kp = Equilibrium steady determined from the incomplete tensions.







