Table of Contents
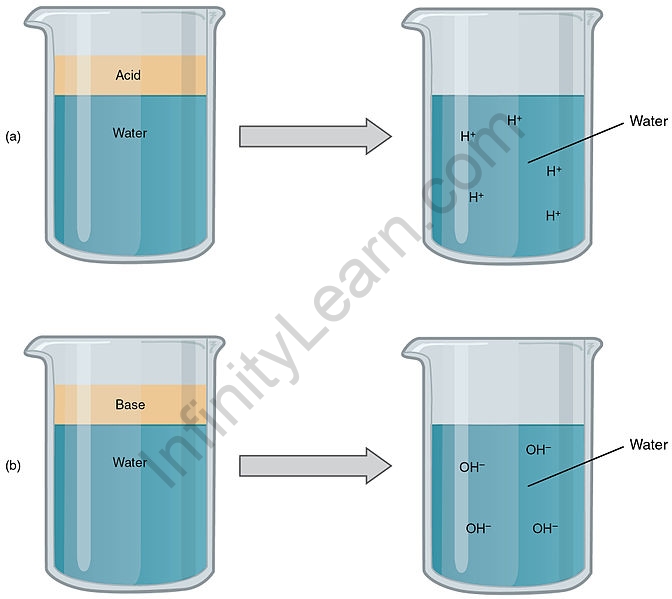
Bases seem to be ionic compounds that, when dissolved in water, produce negative hydroxide ions. An ionic compound is one that contains a negative nonmetal ion as well as a positive metal ion and is held together by an ionic bond. A base, as well known as alkaline, is any substance that reacts with water to produce OH–. Bases in water solutions typically have a pH greater than 7. Because of the self-ionisation of water, there are always some H+ and OH– ions in the solution. There may be more OH– than H+ in simple solutions. Bases that are more focused may have a higher OH– in response. According to the pH scale, a more primary solution—one with more OH– will have a lower pH cost. Acids could indeed react with Bases in a neutralisation reaction, in which the H+ from the acid reacts with the OH– of the bottom to produce a solution with less OH– attention – and a lower pH – than the original Base solution. One of most common inorganic bases are hydroxides (compositions containing OH–), such as sodium hydroxide, NaOH, or calcium hydroxide, Ca(OH)2. Even so, there are a few organic bases, including ammonia-based total molecules like amines, that do not contain OH– at the same time: any OH– in a solution of these molecules is formed through a reaction with water.
 Overview
Overview
Acids and bases play a significant role in biochemistry as well. The pH of the environment affects many biological processes. Controlling acidity in the body is therefore critical. A few biologically significant molecules, such as amino acids, have two personalities. They are amphoteric, which means they can function as both acids and bases. A carboxylic acid group that can produce hydrogen ions and an amino group that can accept hydrogen ions are both present in amino acid. Acids and bases are everywhere, and their uses and properties vary greatly. However, they all involve the movement of hydrogen ions.
A strong base is indeed a fully ionic substance such as sodium hydroxide or potassium hydroxide. In solution, the compound is completely broken down into metal ions and hydroxide ions. Every mole of sodium hydroxide dissolved yields one mole of hydroxide ions in solution. A few strong bases, such as calcium hydroxide, are insoluble in water. That just doesn’t matter because whatever does dissolve is completely ionised into calcium ions and hydroxide ions. Because of the complete ionisation, calcium hydroxide remains a strong base.
Strong Base
In an acid-base reaction, a strong base is a basic chemical compound that can remove a proton (H+) from (or deprotonate) a molecule of even a very weak acid (such as water). Hydroxides of alkali metals and alkaline earth metals, such as NaOH and Ca(OH)2, are common examples of strong bases. Some bases, such as alkaline earth hydroxides, can be used despite their low solubility when the solubility factor is ignored. The fact that “many antacids were suspensions of metal hydroxides such as aluminium hydroxide and magnesium hydroxide” is one advantage of this low solubility.
- Lithium hydroxide
- Sodium hydroxide
- Potassium hydroxide
- Rubidium hydroxide
- Caesium hydroxide
- Magnesium hydroxide
- Calcium hydroxide
- Strontium hydroxide
- Barium hydroxide
- Tetramethylammonium hydroxide
- Guanidine
These strong base cations are found in the first and second groups of the periodic table (alkali and earth alkali metals). Tetraalkylated ammonium hydroxides are indeed strong bases because they completely dissociate in water. Guanidine seems to be a special case of a species that is exceptionally stable when protonated, which is why perchloric acid and sulfuric acid are such strong acids.
Acids having pKa values greater than about 13 are considered very weak, while their conjugate bases are strong.
Strong bases seem to be excellent proton (hydrogen ion) acceptors and donors of electrons. Weak acids can be deprotonated by strong bases. Strong base aqueous solutions are slick and soapy. Even so, touching a solution to test it is never a good idea because these bases are caustic. Chemical burns can result from concentrated solutions.
Superbases (Lewis bases)
Because of the extreme weakness of their conjugate acids—stable hydrocarbons, amines, and hydrogen gas—group 1 salts of carbanions (such as butyllithium, LiC4H9, which dissociates into Li+ and the carbanion C4H9–), amides (NH2–), and hydrides (H–) tend to be even stronger bases. Typically, these bases are formed by reacting conjugate acid with pure alkali metals in their neutral state, such as sodium. They have been called superbases because they cannot be kept in an aqueous solution because they will react completely with water, deprotonating it to the greatest extent possible. In the presence of water, for example, the ethoxide ion (the conjugate base of ethanol) will undergo the following reaction:
CH3CH2O− + H2O → CH3CH2OH + OH−
In contrast to weak bases, which exist in equilibrium with their conjugate acids, the strong base completely reacts with water, leaving no trace of the original anion after the base is added to the solution. Other superbases are as follows:
- Butyl lithium (n-BuLi)
- Lithium diisopropylamide (LDA) (C6H14LiN)
- Lithium diethylamide (LDEA)
- Sodium amide (NaNH2)
- Sodium hydride (NaH)
- Lithium bis(trimethylsilyl)amide, ((CH3)3Si)2NLi
Superbases like the ones mentioned above are frequently used as reagents in organic laboratories.
FAQs
What's the strongest base?
The strongest base on the planet is ortho-diethynylbenzene dianion and this superbase has the highest calculated proton affinity (1843 kJ mol-1), beating out a long-standing contender known as lithium monoxide anion.
What is a strong and weak base?
A weak base has been one that only partially dissociates in solution to give ions. A strong base is a kind that completely dissociates into ions in solution. Weak bases just partially dissociate in a solution, whereas strong bases completely dissociate in a solution.
Is CaOH2 stronger than NaOH?
Ca(OH)2 is really a strong base because it completely dissociates in aqueous solution to yield OH- ion and no moles of it remain undissociated within the solution.
Is magnesium hydroxide a strong base?
All of the liquified magnesium hydroxide dissociates into ions. Magnesium hydroxide is a strong electrolyte because the dissociation of this small amount of dissolved magnesium hydroxide is complete. Because of its low solubility, it is a weak base.