Table of Contents
What is Molecular Orbital Theory?
The Molecular Orbital Theory (MOT) is a scientific idea that helps us understand how molecules stick together. It was created by scientists Hund and Mulliken in the 20th century.
Before MOT, there was another theory called the valence-bond theory. But it couldn’t explain some molecules that have bonds between being weak (like a single bond) and strong (like a double bond). These kinds of bonds are seen in molecules that can change their shape, like in some special molecules.
MOT is better because it can explain these situations. It tells us where we’re likely to find electrons around a molecule and matches the shapes of the molecules themselves. This helps us understand how molecules connect and act.
The key features of the Molecular Orbital Theory:
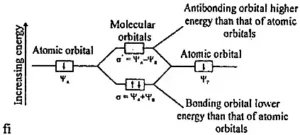
- When atoms come together to form molecules, the number of molecular orbitals they create will match the number of atomic orbitals they had before.
- There are different types of molecular orbitals: bonding ones, anti-bonding ones, and non-bonding ones. Anti-bonding orbitals have higher energy, while bonding ones have lower energy compared to the original atomic orbitals.
- Electrons are placed into these molecular orbitals starting with the lowest-energy orbital and moving up to the higher-energy ones.
- The best combinations of atomic orbitals for making molecular orbitals happen when the original atomic orbitals have similar energies.
In simpler terms, Molecular Orbital Theory tells us that when atoms join together to make molecules, they create new spaces where electrons can be found. These electrons are shared among the atoms in different ways throughout the molecule.
This theory has been really important because it helped us understand how molecules stick together. We think of these molecular orbitals as being made up of atomic orbitals from the individual atoms. We use math and models like the Hartree-Fock or density functional theory to figure out exactly how these orbitals behave based on the Schrödinger equation.
The idea that molecular orbitals in molecules are like mixtures of atomic orbitals can be explained like this:
Video Lesson – Molecular Orbital Theory
Linear Combination of Atomic Orbitals (LCAO)
- Molecular orbitals are like combinations of atomic orbitals, which we call LCAOs. These combinations help us understand how electrons behave when atoms come together to form a molecule.
- We use a math equation called the Schrodinger equation to describe how electrons act in molecular orbitals. It’s similar to how we describe electrons in atomic orbitals.
- This method is like overlapping 2 waves: when they add up nicely, it creates a bonding molecular orbital, but when they cancel each other out, it forms a non-bonding molecular orbital.
Conditions for Combining Atomic Orbitals:
- Similar Energy: When atoms come together to make a molecule, the electron clouds around them should have almost the same amount of energy. It’s like two friends who should be equally strong to hold hands properly.
- Same Shape: The way atoms connect in a molecule should be similar, like two puzzle pieces fitting together perfectly. This way, electrons can be shared between them effectively.
- Good Overlap: The electron clouds from the two atoms should overlap nicely, like two waves meeting and adding up. This creates a strong connection between the atoms.
What Are Molecular Orbitals?
Think of molecular orbitals as invisible spaces around a molecule where we are most likely to find electrons. These spaces are like special shapes that show where electrons hang out in a molecule.
Molecular orbitals are made by mixing the electron clouds of the atoms that make up the molecule. They help us understand how atoms stick together in molecules.
Types of Molecular Orbitals:
According to this theory, there are three main kinds of molecular orbitals:
- Anti-Bonding Molecular Orbitals: These spaces push the atoms apart, weakening the bond between them.
- Non-Bonding Molecular Orbitals: These spaces don’t really affect the bond between the atoms. It’s like they don’t interact much.
Formation of Molecular Orbitals:
When two electron clouds come together, they can either work together (like clapping hands) or cancel each other out (like 2 waves crashing). If they work together, they form a molecular orbital, showing us where electrons are most likely to be around the molecule.
Case 1: When two waves from different atoms match up nicely, they add together, just like when two friends both push a swing at the same time, making it swing higher. This combination is represented as Φ = ΨA + ΨB.
Case 2: When the two waves from different atoms don’t match up, they actually work against each other. It’s like if one friend pushes the swing forward while the other tries to pull it back. This makes a new wave with less amplitude, and we write it as Φ´ = ΨA – ΨB.
Characteristics of Bonding Molecular Orbitals:
- More Electron Attraction: In bonding molecular orbitals, electrons are more likely to be found between the two atoms, which brings the atoms closer together.
- Atoms Attract: The electrons in bonding orbitals make the atoms attracted to each other, like magnets pulling together.
- Stable and Lower Energy: Bonding orbitals are more stable because of this attraction, and they have lower energy compared to the original atomic orbitals.
- Adding Up Waves: They form by adding the atomic orbitals together, creating a stronger wave represented as Φ= ΨA + ΨB.
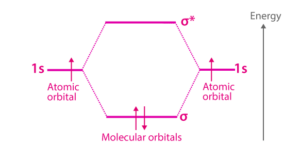
- Notation: We use symbols like σ, π, and δ to represent them.
Characteristics of Anti-bonding Molecular Orbitals:
- Less Electron Attraction: In anti-bonding molecular orbitals, electrons are less likely to be found between the two atoms, pushing the atoms apart.
- Atoms Repel: The electrons in anti-bonding orbitals make the atoms repel each other, like magnets pushing away.
- Less Stable and Higher Energy: Anti-bonding orbitals are less stable because of this repulsion, and they have higher energy compared to the original atomic orbitals.
- Subtracting Waves: They form by subtracting the atomic orbitals, creating a weaker wave represented as Φ´= ΨA – ΨB.
- Notation: We use symbols like σ∗, π∗, and δ∗ to represent them.
Why Are Anti-bonding Orbitals Higher in Energy?
The energy levels of bonding molecular orbitals are always lower because electrons in these orbitals are attracted to the nuclei of the atoms, which brings the atoms closer together. In contrast, anti-bonding molecular orbitals have higher energy levels because electrons in these orbitals experience repulsion between the nuclei of the atoms, pushing the atoms apart. This repulsion results in higher energy and less stability.
Difference between Bonding and Antibonding Molecular Orbitals:
- Bonding orbitals bring atoms closer together, while anti-bonding orbitals push them apart.
- Bonding orbitals are stable and have lower energy, whereas anti-bonding orbitals are less stable and have higher energy.
| Molecular Orbital Theory | |
| Bonding Molecular Orbitals | Anti-Bonding Molecular Orbitals |
| Molecular orbitals formed by the additive effect of the atomic orbitals are called bonding molecular orbitals. | Molecular orbitals formed by the subtractive effect of atomic are called anti-bonding molecular orbitals. |
| The probability of finding the electrons is more in the case of bonding molecular orbitals. | The probability of finding electrons is less in antibonding molecular orbitals. There is also a node between the anti-bonding molecular orbital between two nuclei where the electron density is zero. |
| These are formed by the combination of + and + and – with – part of the electron waves | These are formed by the overlap of + with – part. |
| The electron density in the bonding molecular orbital in the internuclear region is high. As a result, the nuclei are shielded from each other and hence the repulsion is very less. | The electron density in the antibonding molecular orbital in the internuclear region is very low, so the nuclei are directly exposed to each other. Therefore, the nuclei are less shielded from each other. |
| The bonding molecular orbitals are represented by σ, π, δ. | The corresponding anti-bonding molecular orbitals are represented by σ∗ , π∗, δ∗. |
Stabilization and Destabilization Energy:
- Stabilization Energy: This is when atoms coming together in a molecule makes it more stable, like saving money.
- Destabilization Energy: This happens when atoms being together makes the molecule less stable, like spending money.
Paramagnetic and Diamagnetic:
- Paramagnetic: Molecules with unpaired electrons are attracted to magnets, like tiny magnets inside them that align with a magnetic field.
- Diamagnetic: Molecules with all paired electrons are weakly repelled by magnets because the tiny magnets inside cancel each other out.
Now, let’s see which category these molecules fall into:
- B2: Paramagnetic (unpaired electrons).
- C2: Paramagnetic (unpaired electrons).
- O2: Paramagnetic (unpaired electrons).
- NO: Paramagnetic (unpaired electrons).
- CO: Diamagnetic (all electrons are paired).
Features of Molecular Orbital Theory:
- Atoms’ electron clouds combine to create new regions called molecular orbitals.
- Electrons in molecules fill these regions, like how electrons are placed in different energy levels in atoms.
- Molecular orbitals help us understand where electrons are likely to be in a molecule.
- When atomic clouds combine, they should have similar energy levels and shapes.
- The number of molecular orbitals formed equals the number of atomic clouds combined.
- The shape of molecular orbitals depends on the shape of the atomic clouds that combine.
FAQs on Molecular Orbital Theory
What is Molecular Orbital Theory, and how does it apply to you?
F. Hund and R. S. Mulliken established the Molecular Orbital Theory (commonly abbreviated to MOT) to describe the structure and behaviour of various molecules at the beginning of the twentieth century. The valence-bond theory failed to explain how certain molecules, such as those in resonance-stabilized compounds, possess two or more analogous bonds with bond orders that fall between those of a single bond against those of a double bond
What are Molecular Orbitals and How Do They Work?
The molecular orbital function can be used to compute the space in a molecule where the likelihood of finding an electron is highest. The wave behaviour of electrons in a specific molecule is described by molecular orbitals, which are mathematical functions.
What is the significance of MO theory?
By approximating the states of bonded electrons—the molecular orbitals—as linear combinations of atomic orbitals, molecular orbital theory transformed the understanding of chemical bonding (LCAO).