Table of Contents
A nucleophilic addition reaction seems to be an addition reaction in organic chemistry in which a chemical molecule having an electrophilic double or triple bond combines with a nucleophile, breaking the double or triple bond.
A Brief Outline
Nucleophilic additions vary from electrophilic additions in that the group to which atoms are added accepts electron pairs in the former, whereas the latter involves the group donating electron pairs in the latter.
Cyanohydrins are formed when hydrogen cyanide (HCN) reacts with carbonyl compounds (mostly aldehydes and ketones) in a nucleophilic addition process. To speed up the process, base catalysts are frequently utilized. The cyanide anion (CN–) is a nucleophile.
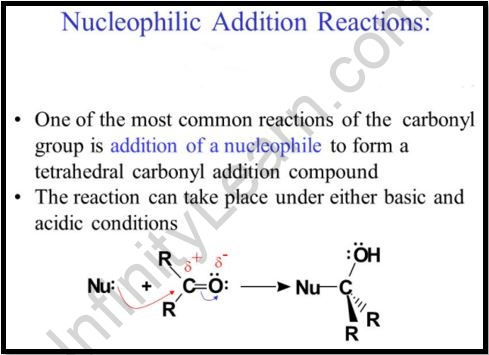
Important concepts
The following three steps can be split down into nucleophilic addition reactions of carbonyl compounds in general.
- The nucleophile and the electrophilic carbonyl carbon form a sigma bond.
- The carbon-oxygen pi link has now been broken, resulting in the formation of an alkoxide intermediate
- The alcohol derivatives are synthesized by the protonation of the alkoxide.
The carbon-oxygen link in carbonyl compounds is polar. The electron density around the oxygen atom is higher due to the oxygen atoms’ significantly higher electronegativity. The oxygen atom obtains a partial negative charge, whereas the carbon atom obtains a slightly positive charge. The carbonyl carbon is an electrophile because it has a partial positive charge. The inclusion of an acidic group can stabilize the partial negative charge on the oxygen atom.
When compared to ketones, aldehydes are more reactive to nucleophilic addition processes. This is because the surrounding R groups stabilize the secondary carbocations generated by ketones. Aldehydes create primary carbocations that are less stable than ketones’ secondary carbocations, making them more vulnerable to nucleophilic assaults.
Significance of Markownikoff and peroxide effect in IIT JEE exam
All 3 primary units of chemistry – organic, inorganic, and physical chemistry – were given similar weightage in terms of high weightage chapters for JEE exams. Organic chemistry is worth 6% of the JEE test and includes topics such as the Markownikoff rule, nucleophilic addition, and reduction processes.
FAQs
Because oxygen is more electronegative in the carbonyl group (C=O), electrons are somewhat pushed towards the oxygen atom. As a result, the carbon atom acquires a partial positive charge (electron-deficient).
The alkyl radicals connected to the carbonyl carbon in ketone have an electron-releasing group (+I effect), which renders the carbonyl carbon less positive. The inclination of carbonyl carbon to embrace the nucleophile is reduced as a result of this.
Hemiacetals are formed via nucleophilic external reactions of aldehydes and ketones with monohydric alcohols. After a reaction with some other molecule of alcohol, the acetal is obtained. Why do nucleophilic reactions occur in aldehydes and ketones but not in carboxylic acid?
Why is it that aldehyde reacts with nucleophiles more quickly than ketones?
What is the nucleophilic addition reaction involving monohydric alcohols and how does it work?



