Table of Contents
Thomson Atomic Model: Why did the Thomson Atomic model proposed by J.J. Thomson failed to explain the atomic structure and its stability? What are the limitations this model? Let us look into the details of the Thomson Atomic model in this article.
Prior to the discovery of subatomic particles, John Dalton proposed Dalton’s atomic theory, which claimed that atoms are indivisible particles. It stated that atoms cannot be broken down further into smaller particles. The discovery of subatomic particles, on the other hand, disproved the postulates proposed in Dalton’s Atomic Theory.
The discovery of subatomic particles prompted researchers to investigate how subatomic particles are arranged in an atom. J.J. Thomson was the first and one of many scientists who proposed models for atomic structure. In 1897, J.J. Thomson discovered negatively charged particles using a cathode ray tube experiment. The particles were given the name electrons.
Electrons, according to J.J Thomson, are two thousand times lighter than a proton. He assumed that an atom is made up of a cloud of negative charges surrounded by a sphere of positive charges. Thomson and Rutherford were the first to demonstrate the ionisation of air in x-rays.
The Thomson atomic model was the first theoretical description of the inner structure of atoms, proposed around 1900 by William Thomson (Lord Kelvin) and strongly supported. In 1897, Sir Joseph John Thomson discovered the electron, a negatively charged part of every atom.
Thomson maintained that atoms are uniform spheres of positively charged matter in which electrons are embedded, despite the fact that Kelvin and others advanced several alternative models in the 1900s.
The plum pudding model, popularly known as the plum pudding model, was abandoned (1911) on theoretical and experimental grounds in favour of the Rutherford atomic model, in which electrons describe orbits around a tiny positive nucleus.
To understand the various chemical reactions that occur around us and the parameters that govern these reactions, we must first understand the elements and compounds that participate in these reactions.
Early scientific discoveries revealed that matter is made up of atoms, which are made up of elementary particles known as electrons, protons, and neutrons.
Overview on Thomson atomic model
William Thomson proposed the Thomson atomic model in the year 1900. This model theoretically explained the description of an atom’s inner structure. Sir Joseph Thomson, who had discovered the electron earlier, was a strong supporter. J.J. Thomson discovered a negatively charged particle during a cathode ray tube experiment.
This experiment was carried out in the year 1897. Cathode ray tubes are vacuum tubes. The negative particle was dubbed an electron.
Thomson assumed that an electron is 2,000 times lighter than a proton and that an atom is composed of thousands of electrons. He considered atoms surrounded by a cloud with both positive and negative charges in this atomic structure model.
He and Rutherford also demonstrated the ionisation of air using X-rays. They were the first to show it. Thomson’s atomic model resembles a plum pudding.
Thomson compared his hypothesised atom model to a Christmas pudding. The electrons in a positively charged sphere were like currants (dry fruits) in a spherical Christmas pudding. We can also think of this as a watermelon, with the positive charge in the atom spread all over like the red edible part of the watermelon, and the electrons embedded in the positively charged sphere like the seeds.
He believed that an atom was made up of thousands of electrons, each of which weighed two thousand times less than a proton. Atoms were surrounded by a cloud with both positive and negative charges in their atomic structure concept. Thomson was successful in explaining an atom’s overall neutrality.
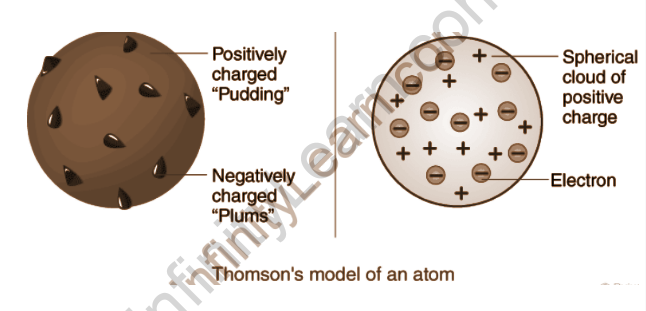
Thomson Atomic Model – Postulates
- Thomson atomic model postulates that an atom resembles a positive-charged sphere with electrons (negatively charged particles) present within the sphere.
- Because the magnitude of the positive and negative charges is equal, an atom has no charge overall and is electrically neutral.
- Thomson’s atomic model is shaped like a spherical plum pudding and a watermelon. It is similar to plum pudding because the electrons in the model resemble the dry fruits embedded in a positive charge sphere, just like a spherical plum pudding.
- The model was also compared to a watermelon because the red edible part of a watermelon was compared to the sphere with a positive charge, and the black seeds filling the watermelon resembled the electrons inside the sphere.
Plum Pudding Atomic Theory
Thomson proposed that the shape of an atom is similar to that of a sphere with a radius of 10-10 m. The positively charged particles are distributed uniformly, and the electrons are arranged in such a way that the atom is electrostatically stable. Thomson’s atomic model was also referred to as the plum pudding or watermelon model. The embedded electrons resembled the seed of a watermelon, and the red mass of the watermelon represented the positive charge distribution. The plum pudding atomic theory assumed that an atom’s mass is uniformly distributed throughout the atom.
Limitations of Thomson Atomic Model
Thomson’s atomic models explained an atom’s overall neutrality. Its assumption that an atom’s total mass is uniformly distributed throughout the atom was inconsistent with some experimental results. Thomson’s atomic model was contradicted by Rutherford’s gold foil experiment of scattering -particles, which revealed that most of the space in an atom is empty.
As a result, it was discovered that the plum pudding atomic model was insufficient to explain atomic structure. Thomson’s atomic model’s limitations prompted additional research into the structure of an atom.
Because the plum pudding atomic model lacked experimental evidence, Rutherford conducted numerous experiments to determine the structure of an atom.
- It failed to explain atomic stability because his atomic model failed to explain how a positive charge holds negatively charged electrons in an atom. As a result, this theory also failed to account for the nucleus’s position in an atom.
- Thomson’s model was unable to account for the scattering of alpha particles by thin metal foils.
- There is no experimental evidence to back it up.
Join JEE 2024 Course from Infinity Learn to make your IIT dream come true.
FAQs
What did JJ Thomson's Atomic Model entail?
J.J. Thomson's experiments with cathode-ray tubes revealed that all atoms contain tiny negatively charged subatomic particles or electrons. Thomson proposed the plum pudding model of the atom, which had negatively charged electrons trapped in a soup of positive effect.
What Is the Meaning of JJ Thomson's Atomic Model?
JJ Thomson's discovery in 1897 was a revolution for the time and a watershed moment in particle physics history. The election was mentioned in the theory for the first time in this model, and the neutrality of the atom was established. The Watermelon model was another name for this model. Despite the fact that this model was not perfect and had some flaws.
Infinity Learn App
Now you can find answers to all your subject queries & prepare for your Exams on our Educational App – Infinity Learn.



