Table of Contents
We understand that the Earth is surrounded by a variety of gases. The Earth is surrounded by a cloud of various gases. It is referred to as air. While air cannot be seen with the naked eye, it can be felt; for example, the presence of air can be felt when we hear the wind blowing. As a result, gases are required for all living organisms to grow and survive. Ideal gases move rapidly and randomly, do not lose energy when they collide, and have no intermolecular forces. This means that when two ideal gas particles come into contact with each other, they are not attracted to each other in the same way that real gas particles are.
Overview
Temperature, volume, and pressure are three variables that can be used to study gases. Volume is maintained constant while studying Gay Lussac’s law, the temperature is kept constant in Boyle’s law, and pressure is kept constant in Charles’s law. The relationship between temperature and pressure of a gas in a fixed volume is defined by Gay Lussac’s law. The propane tanks that we use for barbeque grills are an example of gay Lussac’s law. People use gauges that measure tank pressure to keep track of how much gas is left in the tank. When the air temperature is high, the gauge registers a higher pressure. As a result, before refilling the propane tank, the air temperature must be considered.
When the temperature of a gas sample in a closed container is raised, the pressure of the gas sample rises as well. This is due to the fact that an increase in temperature causes an increase in the kinetic energy of the gas sample, which results in the gas molecules striking the walls of the container with greater force, resulting in higher pressure.
In 1808, Joseph Louis Gay Lussac proposed the Law of Gaseous Volumes. When measured at the same temperature and pressure, the ratio of the volumes of reacting gases is a small whole number, according to this law. This can be thought of as a variant of the law of definite proportions. This law applies to volume, whereas the law of definite proportion applies to mass.
Gay-Lussac’s Law of Gaseous Volume
Whenever the temperature of a sample of gas in a rigid container rises, so does the pressure of the gas. An increase in kinetic energy causes the molecules of gas to strike the container’s walls with greater force, resulting in increased pressure. The relationship between a gas’s pressure and absolute temperature was discovered by the French chemist Joseph Gay-Lussac (1778-1850).
Gay Lussac’s Law states that when the volume is held constant, the pressure of a given mass of gas varies directly with the absolute temperature of the gas. The only difference between Gay-Law Lussac’s and Charles’ Law is the type of container. In a Charles’s Law experiment, the container is flexible; in a Gay-Law Lussac’s experiment, it is rigid.
This gas law is significant because it demonstrates that increasing the temperature of a gas causes its pressure to rise proportionally (assuming the volume does not change). In the same way, as the temperature decreases, the pressure decreases proportionally.
Now, P∝T
That is, P/T=k
Here, P is said to be the pressure exerted by the gas.
T is said to be the absolute temperature of the gas.
k is considered as a constant.
The following graph depicts the relationship between pressure and absolute temperature of a given mass of gas (at constant volume).
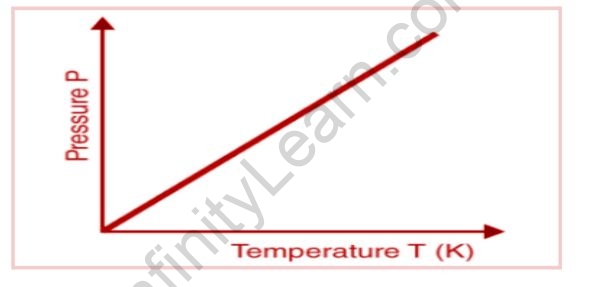
The graph shows that the pressure of a gas (kept at constant volume) constantly decreases as it cools until the gas condenses and becomes a liquid.
Ideal Gas Equation
We Have, P∝T
Then, P=kT…(1)
PV=nRT…(2)
While keeping the value of P from equation (1) to (2) –
kTV=nRT
k=nR/V
k∝1V…(3), which means when volume increases, k will decrease.
Gay Lussac’s Law Examples
- When we heat an aerosol can, the gases change due to the increased temperature. The pressure built up in the can, according to Gay Lussac Law, may result in an explosion.
- Some other examples of heat release are the firing of a bullet. We are all aware that a gun is fired with the assistance of gunpowder. The gunpowder then burns, and the bullet is fired. This is due to the fact that when gunpowder burns, the gases trapped inside are heated. According to Gay Lussac Law, an increase in temperature causes an increase in pressure. As a result of the superheated gases, the bullet is pushed forward with tremendous force towards the barrel of the gun.
- The application of Gay Lussac Law can also be seen in the example of a burning automobile tyre. Heat spreads across the rubber and other constituents of the tyre as it is heated. As a result, the pressure increased (Gay Lussac Law). The increased pressure cannot be absorbed by the tyre. The tyre then explodes because its walls have weakened due to the increased pressure.
- The increased pressure in a pressure cooker while cooking is another example of Gay Lussac Law. As the contents of the pressure cooker heat up, the trapped gases inside, such as steam, exert pressure. This is due to the fact that the temperature of the gases, such as steam, is directly proportional to the pressure exerted. As a result, the steam is released through an external part of the pressure cooker. The faster the food cooks, the higher the temperature and pressure inside the container.
The information about Gay Lussac’s law from various chemistry-related articles is available here. Gay Lussac’s law is an important topic in chemistry. Students who want to pursue their higher studies in chemistry need to be well known about this. It helps them gain deep knowledge about it and also to do well in their exams. The definition, brief explanation, equation, and examples are provided here to assist students in effectively understanding the respective topic. Continue to visit our website for additional chemistry help.
Frequently Asked Questions
What is the importance of Gay Lussac’s law?
The significance of this gas law is that it shows that increasing the temperature of a gas causes a relative increase in its pressure (assuming that the volume does not change). Similarly, decreasing the temperature causes the strain to decrease proportionally.
What is the difference between Gay Lussac’s law and Charles’s law?
All these Gay Lussac's law and Charles' law are ideal gas laws; the main difference is that as per Charles' law, if the pressure remains constant, the volume of a fixed mass of a gas is directly proportional to its absolute temperature. According to Gay Lussac's law, the pressure of a fixed amount of gas at a constant volume varies directly with temperature.
What will happen to the pressure of a closed gaseous system if the temperature increases two-fold?
The pressure is doubled according to Gay Lussac's law.







