Table of Contents
Enthalpy is a function of the state, and its value is determined solely by the system’s initial and final states. The measurement of heat in various reactions is the first step toward understanding thermodynamics. The first thermodynamic principle, the law of energy conservation, seeks to measure the enthalpy or heat of various chemical processes. Every chemical process is linked to a specific amount of enthalpy, which the system receives from its surroundings. Because the reactions occur at constant pressure, the enthalpy change is a convenient way to characterize the reaction. The absolute values of the enthalpies or internal energies are determined by the standard state and compared to the given quantity.
Overview
The most common standard states are gases, liquids, and solids. In molten salt chemistry, different types of reaction enthalpy are observed, such as bonding energy, enthalpy of mixing, enthalpy of dissolution, and enthalpy of polymorphic transformation. The accuracy of estimating the enthalpy of fusion of binary compounds and eutectic mixtures is affected by the volume of input data, the choice of simplifying conditions, the difference among the melting points of the components and binary compounds, and the eutectic temperatures.
The heat absorbed or evolved by the system under constant pressure is equal to the enthalpy change of the system. The total increase in internal energy of the system and the pressure-volume work done can alternatively be characterized as the enthalpy change associated with a process. The enthalpy of the substance or system is the enthalpy stored within the substance or system that is available for conversion into heat.
Enthalpy
Enthalpy is indeed the sum of a thermodynamic system’s internal energy and the product of its pressure and volume. Enthalpy is an energy-like property or state function—it has the dimensions of energy (and thus is measured in joules or ergs), and its value is determined entirely by the system’s temperature, pressure, and composition rather than its history. In symbols, the enthalpy, H, is equal to the sum of the internal energy, E, and the product of the system’s pressure, P, and volume, V: H = E + PV.
The change in internal energy is equal to the heat transferred to the system less the work done by it, according to the law of energy conservation. The enthalpy change is exactly equal to the heat transferred to the system if the only work done is a change in volume at constant pressure. The amount of energy required to change a material’s phase from a liquid to a gas is known as the enthalpy (or latent heat) of vaporization and is expressed in joules per mole. Some phase transitions, such as the enthalpy (or latent heat) of fusion for changing from a solid to a liquid, have similar associated enthalpy changes. As with other energy functions, they are determining absolute values of enthalpy is neither convenient nor necessary. The zero-enthalpy state can be a convenient reference state for each substance. Whenever the temperature rises, so does the number of molecular interactions. When the number of interactions increases, so does the system’s internal energy.
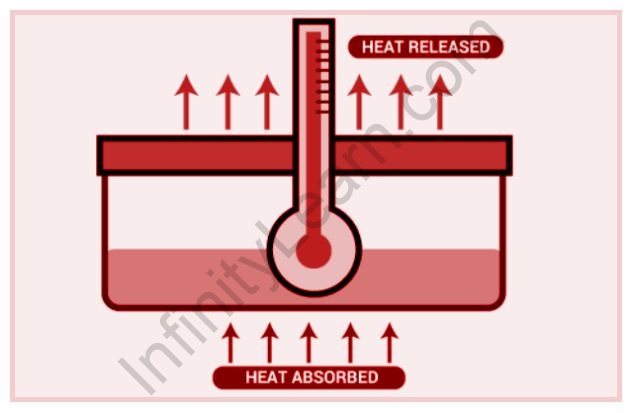
Significance:
- We can tell if a reaction was endothermic (heat absorbed, positive change in enthalpy) or exothermic (heat released, negative change in enthalpy) by measuring the change in enthalpy (released heat, a negative change in enthalpy.)
- It’s used to figure out how much heat a chemical reaction produces.
- In calorimetry, the change in enthalpy is used to measure heat flow.
- A compressor’s minimum power is calculated using enthalpy.
- During a change in the state of matter, enthalpy changes.
- Enthalpy has numerous more applications in thermal engineering.
Sublimation:
Sublimation is not commonly thought of as an analytical approach. It is, however, a method for purifying chemicals or separating mixtures, and as such, it can be useful as a stand-alone procedure or as part of a more complicated analytical method. It can be used with a variety of solids, both inorganic and organic, in a number of matrices and is especially useful when heat-labile materials are included.
Sublimation has been used to manufacture high-purity materials as analytical standards as a method of sample purification. The removal of water from heat-labile materials in the process known as freeze-drying is a specific and widespread example of sublimation employed as a purification method. Below is a more detailed description of the process.
Fractional sublimation has been used as a separation technique to purify samples for analysis by eliminating undesired matrix components or to separate the analyte from the matrix for future analysis.
Transition:
Generally, phase transitions (or phase changes) are the physical processes of transition between one state of a medium, identifiable by specific parameters, and another, with differing values of the parameters, in chemistry, thermodynamics, and many other related sciences. Changes between the basic forms of matter: solid, liquid, and gas, as well as plasma in exceptional situations, are commonly referred to by the name.
A phase of a thermodynamic system, for example, and states of matter both have uniform physical properties. Certain properties of a medium change, frequently discontinuously, during a phase transition as a result of changes in external factors such as temperature, pressure, or others.
Ionization:
In general, ionization is any process that converts electrically neutral atoms or molecules to electrically charged atoms or molecules (ions). We can say that ionization is one of the primary mechanisms through which radiation, such as charged particles and X-rays transfers energy to matter.
When sufficiently intensely charged particles or radiant energy pass through gases, liquids, or solids, ionization occurs. Ionization occurs along the routes of charged particles, such as alpha particles and electrons from radioactive elements. Neutrons and neutrinos are energetic neutral particles that penetrate farther and cause nearly no ionization. The photoelectric effect causes ionization when pulses of radiant energy, such as X-ray and gamma-ray photons, release electrons from atoms. The energetic electrons produced by radiant energy absorption and the passage of charged particles can lead to secondary ionization or further ionization. Because of the continual absorption of cosmic rays from space and UV light from the Sun, the Earth’s atmosphere has a specific minimum amount of ionization.
Enthalpy of Bond Dissociation:
The enthalpy change when one mole of covalent bonds in a gaseous covalent molecule are broken to create products in the gaseous phase is known as bond dissociation enthalpy. Except in the case of diatomic molecules, enthalpy of bond dissociation values differ from bond enthalpy values, which are the average of part of all the bond dissociation energy in a molecule.
Enthalpy of Combustion:
The heat energy released when one mole of a substance burns entirely in oxygen is known as the enthalpy of combustion. The enthalpy change when one mole of a substance is entirely burned in oxygen with all reactants and products in their standard state under standard conditions is known as standard enthalpy of combustion (298K and 1 bar pressure).
Enthalpy of Formation:
Enthalpy of formation is a subset of normal enthalpy of reaction in which two or more reactants combine to produce one mole of product. The enthalpy change when one mole of a compound is created from its constituents in their most stable state of aggregation is known as standard enthalpy of formation (stable state of aggregation at temperature: 298.15k, pressure: 1 atm). Enthalpy of formation is a subset of normal enthalpy of reaction in which two or more reactants combine to produce one mole of product.
Enthalpy of Atomization:
When a compound’s bonds are broken, and the component atoms are divided into individual atoms, the enthalpy of atomization is the amount of enthalpy change. The enthalpy of atomization of an elemental solid is the same as the enthalpy of sublimation of any elemental solid that evaporates into a monatomic gas.
Because the conventional enthalpy change is predicated only on the creation of one mole of gaseous atoms, only half a mole of molecules will be required to convert a diatomic element to gaseous atoms. The computation becomes more difficult when the atoms in the molecule are different isotopes of the same element. The enthalpy change when 1 mole of a substance is entirely split into atoms under normal conditions is called standard enthalpy of atomization (298.15K, 1 bar).
Enthalpy of Sublimation:
The heat required to shift one mole of a substance from solid to gaseous state at a certain combination of temperature and pressure, usually standard temperature and pressure, is known as the enthalpy of sublimation (STP). The heat of sublimation is normally stated in kJ/mol, although it can alternatively be expressed in kJ/kg.
Enthalpy of Phase Transition:
In general, a phase change occurs when a substance transitions from one phase to another. Adding or removing heat at a specific temperature, known as the melting point or boiling point of the substance usually causes the change. The melting point of a substance is the temperature at which it transitions from solid to liquid (or from a liquid to a solid). The temperature at which a substance changes from a liquid to a gas is known as the boiling point (or from a gas to a liquid). The type of the phase transition is determined by the heat transmission direction. The change in enthalpy caused by delivering energy, often heat, to a specified quantity of a substance to change its state from solid to liquid at constant pressure is known as enthalpy of transition.
Enthalpy of Hydration:
We can say that the change in enthalpy when one mole of gaseous ion dissolves in a sufficient amount of water to generate an infinitely dilute solution under a typical condition of 1 bar pressure is known as hydration enthalpy (infinite dilution means a further addition of solute will not cause any heat change).
Enthalpy of hydration is defined as the quantity of energy generated when one mole of gaseous ions is diluted. It can be thought of as the enthalpy of solvation, with water as the solvent. It is commonly known as hydration energy and is always negative.
Enthalpy of Ionization:
The ionization enthalpy is the amount of energy necessary to remove the most loosely bound electron from an isolated gaseous atom and convert it to a gaseous cation. Ionization energy, on the other hand, is the amount of energy required to eject an electron from its ground state to infinity.
Enthalpy of Solution:
In general, a solution is a homogenous mixture of two or more substances that can exist in one of three states: gas, liquid, or solid. The enthalpy of solution, enthalpy of dissolution, or heat of solution is the enthalpy change associated with the dissolving of a substance in a solvent at constant pressure, resulting in infinite dilution. At constant temperature, the enthalpy of a solution is usually given in kJ/mol.
Crack NEET with Result-Oriented Learning Program from Infinity Learn
Frequently Asked Questions
What is enthalpy?
In science and engineering, enthalpy is a term that is used to quantify heat and function. As a material alters under continual strain, enthalpy tells us how much heat and effort has been added to or withdrawn from it. Enthalpy is similar to energy but not the same.
What do you mean by the enthalpy of formation?
The typical reaction enthalpy for the production of a compound from its elements (atoms or molecules) at the given temperature (298.15K) and pressure (1 bar) in their most stable reference states is called formation enthalpy.
What is the difference between the standard enthalpy of reaction and standard enthalpy of formation?
The transition in enthalpy that occurs when one mole of a compound is formed from its constituents is referred to as enthalpy. In a system where a chemical reaction changes one mole of matter, the typical reaction enthalpy occurs.
Infinity Learn App
Now you can find answers to all your subject queries & prepare for your Exams on our Educational App – Infinity Learn.







