Table of Contents
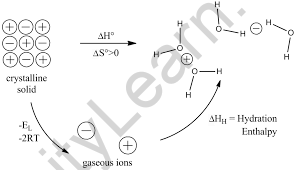
Hydration energy, also known as hydration enthalpy, is the energy generated when one mole of ions undergo hydration, a kind of solvation. It is a type of dissolution of energy in which the solvent is water.
When we dissolve salt in water, the outermost ions (those at the lattice’s border) travel away from the lattice and become covered by nearby water molecules. The salt is known to be water-soluble if the hydration energy is equal to or greater than the lattice energy. Solvation happens with a release of energy in the form of heat in salts where the hydration energy is known to be greater than the lattice energy. When CaCl2 (anhydrous calcium chloride) dissolves, it warms the water. The hexahydrate, CaCl2.6H2O, on the other hand, cools the water upon dissolution. The latter occurs because the hydration energy does not entirely overcome the lattice energy, and the remainder must be extracted from the water to compensate for the energy loss.
The enthalpy of hydration, Hhyd, is the amount of energy released when a mole of an ion dissolves in a vast volume of water, generating an infinitely dilute solution in the process, Az+(g) + aH2O → Az+(aq), where Az+(aq) represents ions surrounded by water molecules and scattered throughout the solution. The estimated hydration energies of several common ions are shown below. According to the table, when the atomic number increases, the ionic size reduces, resulting in a drop in absolute enthalpy of hydration values.
Solution Enthalpy Change
The enthalpy change of solution is defined as the enthalpy change that occurs when one mole of an ionic compound dissolves in water to form an infinitely diluted solution. The solution’s enthalpies might be either negative or positive. In other words, certain ionic compounds dissolve endothermically (for example, NaCl), whereas others dissolve exothermically (for example, NaOH).
An endlessly dilute solution is one in which there is such a massive surplus of water that adding more causes no more heat to be absorbed or generated. As a result, when 1 mole of sodium chloride crystals is dissolved in excess water, the enthalpy change of solution is +3.9 kJ mol-1. The transition is slightly endothermic. As a result, the temperature of the solution will be somewhat lower than the temperature of the original water.
Factors Influencing Hydration Enthalpy Size
Enthalpy of hydration: Hydration enthalpy is the amount of energy generated when positive or negative ions form attractions with water molecules. There may be loose ion-dipole interactions between the – oxygen atoms in the water molecules and the positive ions when there are positive ions, or there may be formal dative covalent (coordinate covalent) connections. Ion-dipole attractions arise between the negative ions and the + hydrogens in water molecules when negative ions are present. The degree of attraction between the ions and the water molecules generally governs the magnitude of the hydration enthalpy. Smaller ions have higher attractions in general. For example, as we move down the Periodic Table, the hydration enthalpies decrease.
Why does the group’s hydration enthalpy decrease?
The greater the hydration enthalpy, the smaller the ion since smaller atoms can accommodate many water molecules surrounding them and get hydrated. The hydration enthalpy falls as one moves down the group, but the atom’s size grows owing to the addition of more valence shells.
In addition, when cation size grows, the hydration enthalpy falls. On the other hand, lattice enthalpy drops quicker than hydration enthalpy owing to the square factor. As a result, the solubility of Group 2 hydroxides rises as one moves down the group.
Factors Affecting Hydration Enthalpy
The quantity the ions are attracted to the water molecules affects the typical enthalpy change of hydration (Hhyd).
The ionic charge and radius are two parameters that influence this attraction.
Ionic Radius
With decreasing ionic radii, ionic radius Hhyd becomes increasingly exothermic.
Smaller ions have a higher charge density, which results in stronger ion-dipole attractions between the water molecules and the ions in the solution.
As a result, they release more energy as they get hydrated, and Hhyd becomes more exothermic.
For example, the hydrogen sulphate (Hhyd) of magnesium sulphate (MgSO4) is more exothermic than the hydrogen sulphate (Hhyd) of barium sulphate (BaSO4)
- Because both compounds include a sulphate ion, the difference in Hhyd must be attributed to the magnesium ion in MgSO4 and the barium ion in BaSO4.
- Magnesium is a Group 2 element and a Period 3 element. Barium is a Group 2 element and a Period 6 element.
- This implies that the Mg2+ ion is less massive than the Ba2+ ion.
- As a result, the attraction for the Mg2+ ion is substantially greater.
- As a result, MgSO4’s typical enthalpy of hydration is more exothermic than BaSO4’s.
Ionic charge
- For ions with higher ionic charges, Hhyd is more exothermic.
- Ions with significant ionic charges have a higher charge density, resulting in stronger ion-dipole interactions between water molecules and ions in solution.
- As a result, they release more energy as they get hydrated, and Hhyd becomes more exothermic.
- The Hhyd of calcium oxide (CaO), for example, is more exothermic than the Hhyd of potassium chloride (KCl).
Hydration Enthalpy Application
The reaction of cement with water is one use of enthalpy of hydration. Because the process is exothermic, it generates a lot of heat. This heat generated becomes substantial in large-scale projects such as dams and other buildings. Large amounts of cement are utilized in the building of enormous concrete blocks.
Heat is emitted during the setting process. The exterior surfaces of the block cool comparatively quicker than the interior, creating a thermal gradient in the block and potentially initiating fractures that lead to structural collapse.
To prevent this difficulty, low heat cement is favoured for large-scale buildings, as is cement containing pozzolanic admixtures such as fly ash or slag, and ice instead of water to produce concrete.
Importance of the topic Hydration Enthalpy for JEE & CBSE Exam
Hydration Enthalpy is an important topic for JEE Exam Preparation. Students can score better marks by developing an understanding of this topic.
FAQ’s
Is the enthalpy of hydration positive or negative?
The enthalpies of hydration are always negative. Hydration enthalpy is the amount of energy generated when positive or negative ions attach water molecules.
What is the link between the enthalpy of hydration and solubility?
When the hydration enthalpy is large, the chemical is very soluble in water.
Is Hydration Absorbing or Releasing Energy?
Hydration enthalpy, also known as Hydration energy, is the amount of energy released during the hydration of a single ion-molecule. Drainage capacity is one component of the solution volume analysis. It is a unique water condition.








