Table of Contents
Introduction:
An orbital is a mathematical statement that explains the behavior of no more than two electrons in the proximity of an atomic nucleus or a system of nuclei such as a molecule in chemistry and physics. An orbital is frequently depicted as a three-dimensional region in which the electron has a 95% chance of being found. Atomic orbitals are commonly denoted by a combination of numerals and letters representing specific properties of the electrons associated with the orbitals, such as 1s, 2p, 3d, and 4f. The digits, known as principal quantum numbers, represent energy levels as well as the relative distance from the nucleus. A 1s electron lives in the energy level closest to the nucleus. A 2s electron, which is less strongly bound, spends the majority of its time away from the nucleus.
The orbital shape is denoted by the letters s, p, d, and f. (The shape is determined by the magnitude of the electron’s angular momentum as a result of its angular motion.) The nucleus is at the center of a s orbital, which is spherical. A 1s electron is thus almost entirely confined to a spherical region close to the nucleus, whereas a 2s electron is restricted to a slightly larger sphere. A p orbital is roughly the shape of a pair of lobes on opposite sides of the nucleus or a dumbbell shape. An electron in a p orbital has the same chance of being in either half.
The other orbitals have more complicated shapes. Before the relationship between spectra and atomic electron configuration was known, the letters s, p, d, and f were used to classify spectra descriptively into series called sharp, principal, diffuse, and fundamental. There are no p orbitals in the first energy level, but there are three in each of the higher levels. These triplets are oriented in space as if they were on three axes at right angles to one another and can be distinguished by subscripts such as 2px, 2py, and 2pz.
There are five d orbitals in all but the first two principal levels and seven f orbitals in all but the first three principal levels, all with complicated orientations. Because of their spin, each orbital can only have two electrons. An electron can be thought of as having either a clockwise or counterclockwise spin about its axis, transforming it into a tiny magnet. Electrons in full orbitals are paired with spins or magnetic polarities that are opposite.
Overview:
An orbital is a mathematical function that describes the wave-like behavior of an electron, electron pair, or (less commonly) nucleons in chemistry and quantum mechanics. An orbital is also known as an atomic orbital of an electron orbital. Although most people think of an “orbit” as a circle, probability density regions that may contain an electron can be spherical, dumbbell-shaped, or more complicated three-dimensional shapes. The mathematical function’s goal is to map the likelihood of an electron being found in a region around (or potentially inside) an atomic nucleus.
An electron cloud with an energy state specified by given values of the n, l, and ml quantum numbers is referred to as an orbital. Each electron is described by a set of quantum numbers that are unique to it. An orbital is a portion of an atom that can contain two electrons with paired spins and is commonly connected with that region. The s, p, d, and f orbitals are orbitals with angular momentum quantum numbers of 0, 1, 2, and 3, respectively. The letters s, p, d, and f derive from descriptions of alkali metal spectroscopy lines as sharp, principal, diffuse, or fundamental. Beyond l = 3, orbital names are alphabetical afters, p, d, and f. (g, h, i, k, …). The letter j is omitted because it is identical to the letter I in all languages.
Concept of orbital numbers
Orbital numbers can be used to describe an electron’s trajectory and movement within an atom. The Schrodinger equation must be satisfied when the quantum numbers of all the electrons in a given atom are combined together. The collection of numbers used to characterize the position and energy of an electron in an atom is known as quantum numbers.
Quantum numbers are divided into four categories: primary, azimuthal, magnetic, and spin.
An atomic orbital is a wave function for an electron in an atom that describes a region of space where the electron has a high probability of being found. Energy changes within an atom occur as a result of an electron switching from one energy wave pattern to a different energy wave pattern (usually accompanied by the absorption or emission of a photon of light).
Four different quantum numbers describe each electron in an atom. The first three (n, l, ml) specify the orbital of interest, while the fourth (ms) specifies the number of electrons that can occupy that orbital.
Types of orbitals
An atom contains a large number of orbitals. If an orbital is smaller in size, it means that the electron is more likely to be found close to the nucleus. Similarly, for the shape, there is a greater possibility of finding electrons along with certain directions than others.
S-Orbital
The s orbital is a spherically symmetrical orbital that revolves around the atomic nucleus. As we move away from the nucleus, the energy level rises, and the orbitals grow larger. Sizes are listed in the following order: 1st, 2nd, and 3rd. The probability of finding an electron is greatest in 1s and rapidly decreases as we move away from it. In the case of the 2s orbital, the probability density drops sharply to zero before increasing again. When it reaches a small maximum, it decreases again until it reaches zero if the value of r is increased further.
The nodal point is a point where there is no chance of finding the electron. Nodes are classified into two types: radial nodes and angular nodes. The distance from the nucleus is calculated by the radial nodes, while the direction is determined by the angular nodes. The number of radial nodes is equal to n – l – 1.
l = number of angular nodes
n – 1 represents the total number of nodes.
Nodal planes are defined as planes with a zero probability of finding an electron. The number of planes is the same as l.
P-Orbital
The p orbitals are shaped like dumbbells. The p orbital node is located in the nucleus’s center. The p orbital can hold a maximum of six electrons due to the presence of three orbitals. The three p orbitals are at right angles to one another. The size of the p orbitals is determined by the principal quantum number n, with 4p being larger than 3p and 2p being smaller.
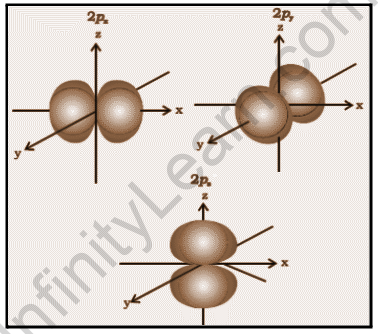
Each p orbital has sections known as lobes on either side of the plane that runs through the nucleus. At the plane where the two lobes intersect, the probability of finding an electron is nil. Because the three orbitals are the same size, shape, and energy, they are referred to be degenerate orbitals. The only difference between the orbitals is the orientation of the lobes. Because the lobes are oriented along the x, y, or z-axis, they are given the designations 2px, 2py, and 2pz. The number of nodes is calculated using the formula n –2.
D-Orbital
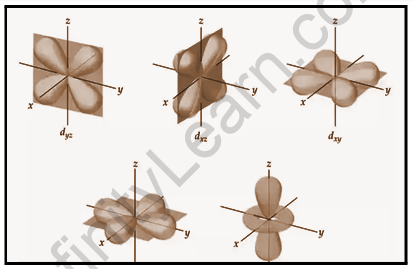
The d orbital is represented by a cloverleaf or two dumbbells in a plane. Because the value of l=2 for the d orbital, the minimum value of the principal quantum number n is 3. The value of l must be less than or equal to n-1. For l = 2, the values of ml corresponding to d orbitals are (–2, –1, 0, +1, and +2), implying that there are five d orbitals.
The five d-orbitals are denoted by the letters dxy,dyz,dxz,dx²–y², and dz². The energy of all five orbitals is the same, but the first four orbitals are similar in shape, whereas the dz² differs from the others. The probability density function (radical node) is zero. The dxy orbital has two nodal planes that pass through the origin and bisect the xy plane, which includes the z-axis. For d orbital, there are two angular nodes.
F-Orbits
The shape of the f orbital is diffused. Because the value of l=3 for the f orbital, the minimum value of the principal quantum number n is 4. The corresponding ml values for the f orbital are (-3,–2, –1, 0, +1, +2, +3). As a result, with l = 3, there are seven f orbitals.
The seven orbitals are as follows:
fx(x²-y²),fy(x²-y²),fxyz’,f³z,fyz‘,fxz²,fz(x²-y²)

In Quantum Mechanics and Chemistry, an orbital is a mathematical function that illustrates the wave-like behavior of an electron pair, electron, or nucleon. Orbitals are also known as electron orbitals or atomic orbitals. Atomic orbitals are three-dimensional regions of space surrounding an atom’s nucleus. Atomic orbitals enable atoms to form covalent bonds. The most commonly filled orbitals are s, p, d, and f. According to the Pauli Exclusion Principle, no orbital space can contain more than two electrons.
Also read: Important Topic Of Physics: Quantum Numbers
FAQs:
In chemistry, how many orbitals are there?
The four different orbital forms (s, p, d, and f) have different sizes, and one orbital can only hold two electrons. Because the orbitals p, d, and f have separate sub-levels, they can hold more electrons. Each element's electron configuration is unique to its position on the periodic table, as shown.
How do orbitals function?
In atomic theory and quantum mechanics, an atomic orbital is a mathematical term that describes the wave-like behavior of one electron or a pair of electrons in an atom. Each orbital can only hold two electrons at a time, each with its unique amount of spin.
What is the total number of orbitals?
Because the s sublevel has only one orbital, only two electrons can be present. Because the p sublevel has three orbitals, a maximum of six electrons can be present. Because the d sublevel has 5 orbitals, a maximum of 10 electrons can be present. And each of the four sub-levels has seven orbitals that can hold a maximum of 14 electrons.







