Table of Contents
A double bond seems to be a covalent link between two atoms that involves four bonding electrons rather than the two that are involved in a single bond. Alkenes, in particular, do have a lot of double bonds between two carbon atoms. Many double bonds exist between two different elements, for as between a carbon atom and an oxygen atom in a carbonyl group. Azole compounds (N=N), imines (C=N), and sulfoxides (S=O) are examples of common double bonds. A double bond is depicted as two parallel lines (=) between the two connected atoms in a skeleton formula; the equals sign is used typographically for this. Alexander Butlerov, a Russian scientist, first used the double bonds in chemical nomenclature.
A brief outline
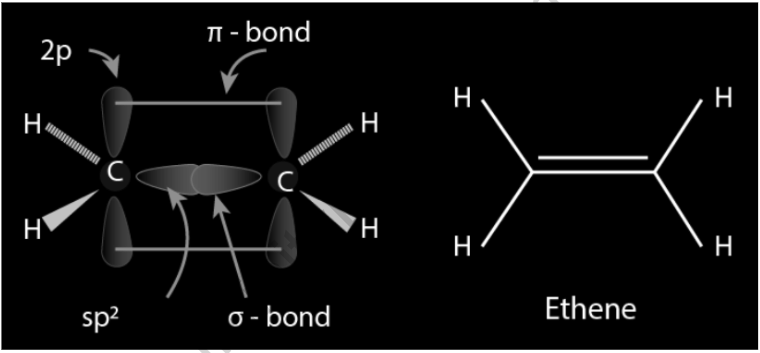
C2H4 Double Bond: Orbital hybridization can be used to explain the type of bonding. Each carbon atom in ethylene has three sp2 orbitals and one p orbital. The three sp2 orbitals are arranged in a plane with 120° angles between them. This plane is perpendicular to the p-orbital. Two of the sp2 orbitals overlap when the carbon atoms approach each other, forming a sigma bond. The two p-orbitals approach (again in the same plane) at the same time, forming a pi bond. Because the p-orbitals must remain parallel for maximum overlap, rotation around the core bond is not conceivable. Cis-trans isomerism is caused by this feature. Because p-orbital overlap is maximized, double bonds are shorter than single bonds.
The C=C bond length in ethylene is 133 pm, which is shorter than the C=C bond length in ethane, which is 154 pm. The double bond is likewise stronger than the sigma bond, with 636 kJ mol1 versus 368 kJ mol1, although not by nearly as much as the pi-bond, which is weaker due to less effective pi-overlap. As with a bent bond, the double bond is formed by two overlapping sp3 orbitals in another picture.
Important concepts
Bonding in Ethene (double bond)
- So far, the Valence Bond Theory has been able to explain bonding in molecules with single bonds. However, when molecules have double or triple bonds, the model requires more data.
- Ethene (also known as ethene) is the simplest chemical having a carbon-carbon double bond, CH2=CH2. According to its Lewis structure, ethene comprises one carbon-carbon double bond and four carbon-hydrogen single bonds.
- In one experiment, the four carbon-hydrogen bonds in the ethene molecule were shown to be identical. According to VSEPR theory, the molecule should have a trigonal planar shape since each carbon is encircled by three electron groups.
- One bond looks to be distinct, regardless of the fact that each carbon has fulfilled its tetravalent criteria. This is clearly a different type of orbital.
Use of ethene
The use of ethylene can be divided into two categories:
1) as a monomer from which longer carbon chains can be constructed, and 2) as a catalyst for the formation of long carbon chains.
2) as a starting point for the formation of other two-carbon compounds.
The first is the single largest consumer of ethylene, accounting for over half of the annual output. Polymerization (the time-consuming combining of many small particles into larger ones) of ethylene produces polyethylene, a polymer with diverse applications, including bundling films, wire coatings, and crush bottles.
Ethene’s Applications
- This method is used to make a variety of important polymers, including polyethylene and polyvinyl chloride (PVC). These polymers are used in raincoats, shoe soles, pipelines, and floor tiles.
- Ethene is largely utilized in polymer synthesis. Poly(ethene) accounts for over 60% of global ethene demand (HDPE 28%, LLDPE 18%, LDPE 14%), with dichloro-1,2-ethane, the precursor for chloroethene and hence PVC, accounting for the remaining 11%. Another 5% is used to create ethylbenzene, which is utilized in the production of polymers (phenylene).
- Epoxyethane is manufactured from around 16% of ethene in the world (ethene oxide).
- Various large polymers, such as polyethylene and polyvinyl chloride, are collected (PVC). Waterproof shells, shoe soles, lines, and floor tiles are all made with these polymers.
- To organize epoxyethane, a chemical that is employed in the development of new products.
- Terylene is used to create ethyl glycol, which is then used to organize Terylene.
- To use ethyl alcohol (C2H5OH), acetaldehyde (CH3CHO), and other large manufactured compounds as a broad narcotic.
Double benzene ring
The chemical compound naphthalene has the formula C10H8. It’s the simplest polycyclic aromatic hydrocarbon, and it’s a white crystalline solid with a distinct odour discernible at concentrations as low as 0.08 parts per million by mass. The structure of naphthalene is that of an aromatic hydrocarbon is comprised of a bonded pair of benzene rings. It’s well recognized for being the major ingredient in mothballs. A naphthalene molecule is made up of two benzene rings fused together. (In organic chemistry, rings that share two or more atoms are said to be fused.) As a result, benzenoid polycyclic aromatic hydrocarbons like naphthalene are classed as benzenoid polycyclic aromatic hydrocarbons (PAH)
All of the heavier group 14 elements now have double bonded compounds, alkene homologs, and R2E=ER2. These compounds, unlike alkenes, do not have planar structures and instead have distorted and/or trans-bent structures. For the heavier elements, these effects become much more pronounced. Two weak donor-acceptor bonds are formed, with the lone pair on each tin atom overlaying the vacant p orbital on the other.
Significance of ethene in NEET exam
Chemistry necessitates a deep comprehension of ideas in order to pass board and NEET exams. Preparing for a subject without enough study materials and solutions, on the other hand, can be challenging. As a result, referring to NEET chemistry solutions or practicing with these resources will assist students in achieving good scores. You can get a good grade if you prepare well for the exam with Infinity Learn. It is recommended that you read all of the chapters and revise them on a frequent basis. Strengthen your fundamentals through practice. Furthermore, rather than mastering every detail of the syllabus, it is preferable to concentrate on the most important areas.
FAQs
There are only four C-H bonds, one C=C bond, and no lone pairs on the last shells, according to the Lewis structure of C2H4. Among a carbon atom and a hydrogen atom, there is only one bond. Furthermore, in ethene C2H4, there is only one double bond.
Ethene isn't a particularly complicated molecule. Apart from this, ethene's carbon atoms are doubly bound to each other, and each carbon atom is likewise bonded to two hydrogen atoms. They unite to make three bonds to each carbon atom, resulting in sp2 Hybridization.
Because a double bond is formed and each carbon is connected to three atoms, the geometric shape of ethene is a trigonal planar shape, which implies two overlapping triangles are present. In ethene C2H4, however, many single and double bonds are there?
What is the nature of ethene's double bonds?
What is the shape of ethene?





