General Principles and Processes of Isolation of Elements Class 12 Chemistry MCQs Pdf
1. The electrolytic reduction technique is used in the extraction of
(a) Highly electronegative elements.
(b) Highly electropostive elements.
(c) Metalloids.
(d) Transition metals.
Answer
Answer: b
2. In the commercial electrochemical process for aluminium extraction, electrolyte used is
(a) Al(OH)3 is NaOH solution.
(b) An aqueous solution of Al2 (SO4)3.
(c) A molten mixture of Al2O3 and Na3AlF6.
(d) A molten mixture of Al2O3 and Al(OH)3.
Answer
Answer: c
3. Which ore can be best concentrated by froth floatation process?
(a) Malachite
(b) Cassiterite
(c) Galena
(d) Magnetite
Answer
Answer: c
4. Electrolytic reduction of Al2O3 to Al by Hall- Herault process is carried out
(a) in presence of NaCl.
(b) in presence of fluorite.
(c) in presence of cryolite which forms a melt with lower melting point.
(d) in presence of cryolite which forms a melt with high melting point.
Answer
Answer: c
5. The chemical composition of ‘slag’ formed during the melting process in the extraction of copper is
(a) Cu2O + FeS
(b) FeSiO3
(c) CuFeS2
(d) Cu2S + FeO
Answer
Answer: b
6. Bessemer converter is used in the manufacture of
(a) Pig iron
(b) Steel
(c) Wrought iron
(d) Cast iron
Answer
Answer: b
7. The method of zone refining of metals is based on the principle of
(a) greater mobility of the pure metal than that of the impurity.
(b) higher melting point of the impurity than that of the pure metal.
(c) greater noble character of the solid metal than that of impurity.
(d) greater solubility of the impurity in the molten state than in the solid.
Answer
Answer: d
8. In the leaching of Ag2S with NaCN, a stream of air is also passed. It is because
(a) The reaction between Ag2S and NaCN is reversible.
(b) to oxidise Na2S formed in the reaction to Na2SO4.
(c) to oxidise Ag2S to Ag2O.
(d) Both (a) and (b).
Answer
Answer: d
9. Purest form of iron is
(a) Cast iron
(b) Hard Steel
(c) Stainless steel
(d) Wrought iron
Answer
Answer: d
10. Consider the following reaction at 1000° C
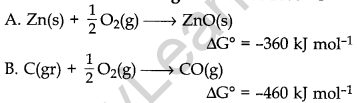
Choose the correct statement at 1000°C
(a) Zinc can be oxidised by carbon monoxide.
(b) Zinc oxide can be reduced by graphite.
(c) Both statements (a) and (b) are correct.
(d) Both statements (a) and (b) are false.
Answer
Answer: b
We hope the given Chemistry MCQs for Class 12 with Answers Chapter 6 General Principles and Processes of Isolation of Elements will help you. If you have any query regarding CBSE Class 12 Chemistry General Principles and Processes of Isolation of Elements MCQs Pdf, drop a comment below and we will get back to you at the earliest.

