Table of Contents
- Reaction of Acids with Water
- Reaction of Bases with Water
- Summary
- What’s Next?
In the previous segment of the chapter ‘Acids, Bases and Salts’, we studied the common properties among all acids and all bases. In this segment, let us understand the reaction of acids and bases with water.
What happens when Acids react with water?
- When hydrochloric acid is added to water to make a solution, it reacts with the water molecules.
- The hydrochloric acid will dissociate into ?+ ??? ??− ions but this happens only in aqueous solutions. The reaction is
???(??) → ?+ + ??−
- The hydrogen ion reacts with water to give the hydronium ion because hydrogen ions cannot exist alone. They only exist after combining with the water molecule. This can be generalized as
?+ + ??? → ???+???
- There are two ways in which we can represent the hydrogen ions, ?+(??)?????+
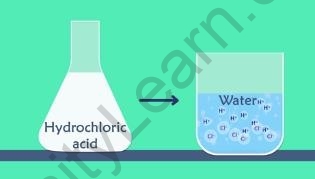
Dissociation into ?+?????−
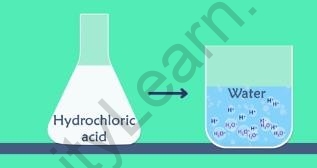
Dissociation into ???+
What happens when Bases react with water?




