Table of Contents
- Reaction of Metallic Oxides with Acids
- Reaction of Non-metallic Oxides with Bases
- Summary
- Did You Know?
- What’s Next?
In the previous segment of the chapter ‘Acids, Bases and Salts’, we studied the reaction of acids with bases. In this segment, let us understand the reaction of metallic oxides with acids and non-metallic oxides with bases.
How do metallic oxides react with Acids?
- Metallic oxides are basic in nature. They react with acids to give salt and water.
- Copper oxide is a metallic oxide.
- When dilute hydrochloric acid is added to a beaker containing a small amount of copper oxide, the copper oxide dissolves. The colour of the mixture becomes bluish-green because of the formation of Copper(II) Chloride.
The reaction taking place in this experiment is
???(?) + ????(??) → ?????(??) + ???(?)
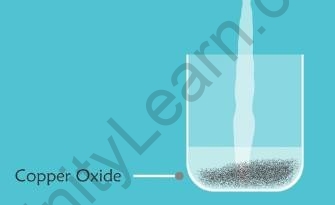
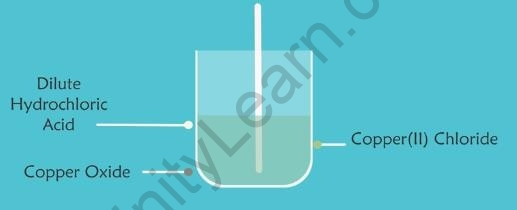
Copper Oxide Green Solution of Copper (II) Chloride
- It is a neutralisation reaction in which the solid copper oxide reacts with hydrochloric acid to produce a soluble salt copper(II) chloride and water.
This can be generalized as
Metal Oxide + Acid → Salt + Water

