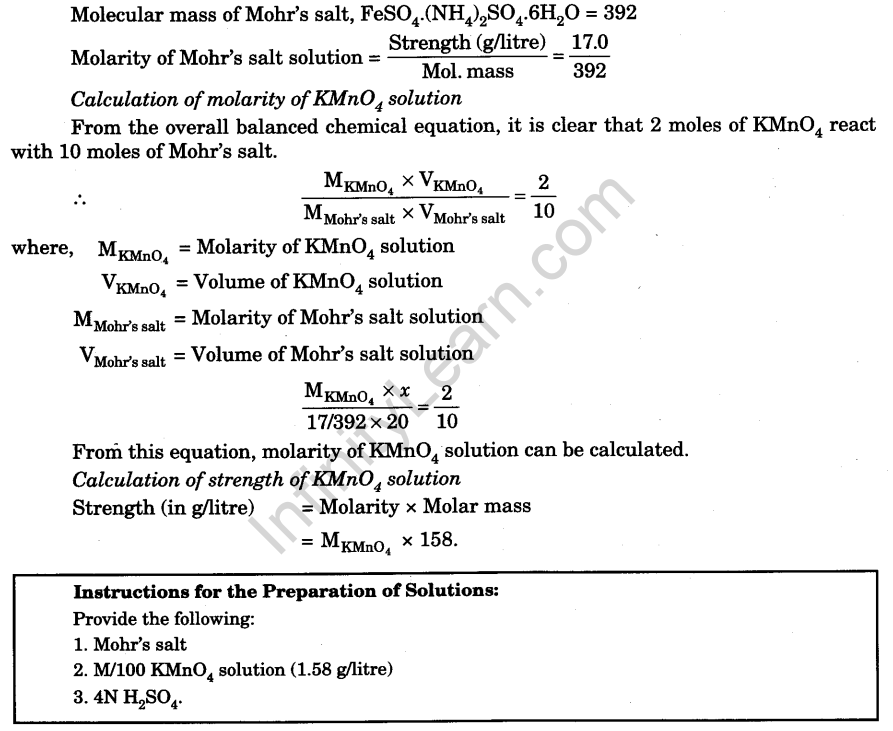
Prepare a Solution of Ferrous Ammonium Sulphate (Mohr’s salt) Containing Exactly 17.0 g of the Salt in one litre. With the help of this Solution, Determine the Molarity and the Concentration of KMnO4 in the Given Solution
Chemical Equations
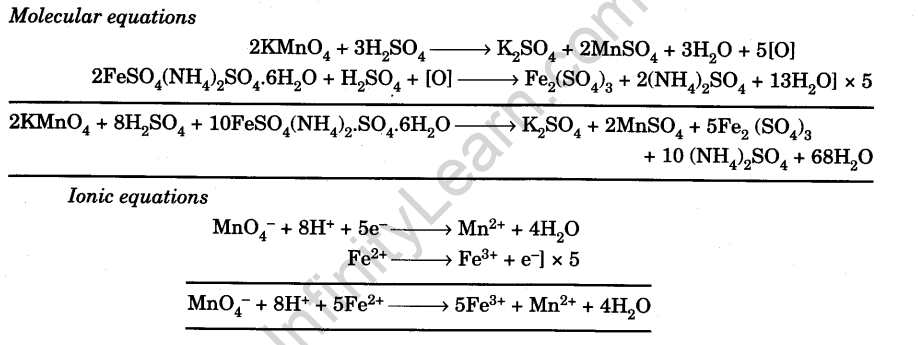
Indicator
KMnO4 is a self-indicator.
End Point
Colourless to permanent pink colour (KMnO4 in burette).
Procedure
1. Weigh exactly 4.250 g of Mohr’s salt on a watch glass and dissolve in water to prepare exactly 250 ml of solution with the help of a 250 ml measuring flask. Rinse the pipette with the prepared Mohr’s salt solution and pipette out 20.0 ml of it in a washed titration flask.
2. Rinse and fill the burette with the given KMnO4 solution.
3. Add one test-tube (~ 20 ml) full of dilute sulphuric acid (~ 2 M) to the solution in titration flask.
4. Note the initial reading of the burette.
5. Now add KMnO4 solution from the burette till a permanent light pink colour is imparted to the solution in the titration flask on addition of last single drop of KMnO4 solution.
6. Note the final reading of the burette.
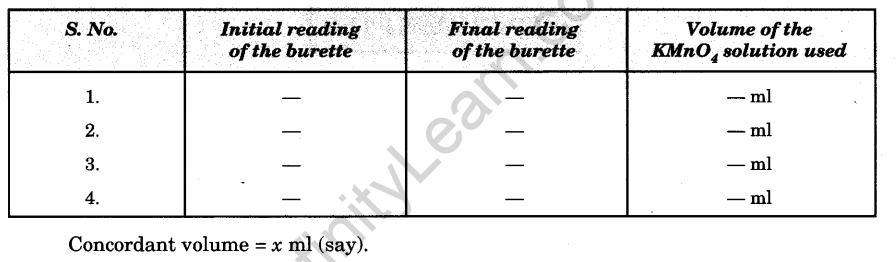
7. Repeat the above steps 4-5 times to get a set of three concordant readings.
Observations
Weight of watch glass =……. g
Weight of watch glass + Mohr’s salt =…………..g
Weight of Mohr’s salt = 4.250 g
Volume of Mohr’s salt solution prepared = 250 ml
Volume of Mohr’s salt solution taken for each titration = 20.0 ml
Calculations
Concentration of Mohr’s salt, ferrous ammonium sulphate, FeSO4.(NH4)2 SO4.6H20 in the prepared solution = 17.0 g/litre.