Table of Contents
Introduction:
Electromagnetic Radiation (EMR) is a type of energy flow in which both electrical and magnetic fields change at the same time. Radio waves, microwaves, infrared light, visible light, ultraviolet light, X-rays, and gamma rays are all examples of electromagnetic radiation. Electromagnetic radiation travels through space and vacuum by oscillating electrical and magnetic fields created by its particles. In the early 1870s, a Scottish physicist named Sir James Clerk Maxwell proposed the theory of Electromagnetic Radiation.
It was experimentally confirmed by Heinrich Hertz, a German scientist. According to Maxwell, when an electrically charged particle accelerates, time alternating electrical and magnetic fields form, facilitating the particle’s propagation.
The information about electromagnetic radiation from various physics-related articles is available here. Electromagnetic radiation and its general concepts are important topics in physics. Students who want to flourish in physics need to be well known about electromagnetic radiation to get deep knowledge about it to do well on their exams. The general ideologies, and concepts related to electromagnetic radiation are provided here to assist students in effectively understanding the respective topic. Continue to visit our website for additional physics help.
Overview:
At light speeds (2.998 108m / s), electromagnetic radiation interferes with electrical and magnetic field movement. It has no mass or charge and travels in a series of photons, or quanta, which are dynamic energy packets. Radio waves and microwaves, as well as infrared, ultraviolet, gamma, and x-rays, are examples of EM rays. Celestial sources (such as the sun and stars), radioactive elements, and man-made gadgets are all examples of EM radiation sources. The dual wave and particle duality of EM are reflected in its name.
Electromagnetic radiation moves at a steady rate in the direction of waves. The relationship between speed and wavelength (one cycle’s straight line distance) and frequency (cycles per second, or hertz, Hz), represented in the formula, reveals EM waves properties.
Electromagnetic radiation is a type of energy created by the flow of electrically charged particles through matter or vacuum, as well as oscillating magnetic and electric disturbances. The magnetic and electric fields intersect at 90°, and the combined waves travel perpendicular to both the electric and magnetic oscillating fields that cause the disturbance.
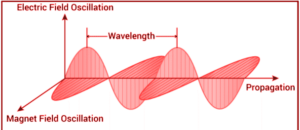
Properties of Electromagnetic Radiation:
Electron radiation is emitted as photons when electromagnetic radiation occurs. These are quantized harmonic waves or bundles of light energy that travel at the speed of light. The energy is then classified into several groups based on the wavelength of the electromagnetic spectrum. Magnetic and electric waves travel perpendicular to each other and have a wavelength, amplitude, and frequency properties. The following paragraphs discuss some basic features of electromagnetic radiation.
- They have the ability to traverse through empty space. Other than electromagnetic waves, all waves must pass through a matter. Sound waves, for example, require the passage of a solid, liquid, or gas.
- Light travels at a constant speed of 2.99792458 x 108m/s.
- The sign ‘’ is widely used to represent wavelength. It’s a measurement of the distance between troughs and crests.
Dual Behaviour of Electromagnetic Radiation:
The photoelectric effect can be described by noting that radiations are made up of microscopic energy packets known as quanta. These energy packets can be thought of as particles. Radiations, on the other hand, display interference and diffraction phenomena, indicating that they are wavelike. As a result, electromagnetic radiation can be said to have a dual nature. That is Particle nature and Wave nature.
Particle Nature of Electromagnetic Radiation:
The photoelectric effect and black body radiation are not explained by the wave theory of radiation. An ideal black body is a perfect radiation absorber and emitter. When a body like this is heated, it turns red. To put it another way, it emits a reddish light. As the temperature rises, the colour of the radiated radiation shifts from red to yellow to white, then purple as the temperature rises even higher. This means that when the temperature rises, the wavelength of the radiation emitted by a dark body decreases.
Planck’s quantum theory:
Planck’s quantum theory defines why light is emitted and absorbed.
Planck’s quantum theory postulates are:
- Matter emits or absorbs energy in discrete amounts, in the form of little packets or bundles, in a discontinuous manner.
- Quantum energy is the tiniest bundle or packet of energy. In the case of light, a photon is a quantum of light.
- The frequency of the radiation is directly proportional to the energy of the quantum absorbed or released.
Planck is widely acknowledged as the founder of quantum mechanics.
We have, E=hv, where h is Planck’s constant (6.62606957(29) x 10-34 J s), v represents frequency, and E is the electromagnetic wave’s energy, according to Planck.
Planck remarked (cautiously) that this was merely a side effect of radiation absorption and emission and had nothing to do with the physical reality of the radiation. However, in 1905, Albert Einstein reinterpreted Planck’s quantum hypothesis to explain the photoelectric effect, which occurs when light shines on specific materials and causes electrons to be expelled.
Currents
Frequently Asked Question (FAQs):
Question 1: Are Electromagnetic Radiation Harmful to Humans?
Answer: There is little doubt that short-term exposure to high levels of electricity can be hazardous to one’s health. So far, there is no reason to believe that low magnetic fields are harmful to human health without more investigation.
Question 2: What is meant by Electric Waves and their structures?
Answer: On a vacuum frequency of 3 x 108m/s, EM waves are mounted. The gravitational force, or electric field, does not deviate. They may, however, express frustration or dissatisfaction. An electric pulse can flow through anything, whether it’s air, a solid object, or a vacuum.
Question 3: How is blackbody radiation produced?
Answer: Because of their temperature, all objects emit electromagnetic radiation. A black body is an idealized object that consumes electromagnetic energy that it comes into touch with. The temperature of the continuum determines the amount of heat radiation released.
Question 4: What is Planck’s constant?
Answer: The Planck constant compares the total energy of a photon to the frequency of its electromagnetic wave. It is named after physicist Max Planck. In quantum physics, it is a fundamental quantity.
Question 5: What is Stefan’s law of radiation?
Answer: Stefan-law Boltzmann states that a surface’s overall radiant heat power is proportional to its fourth absolute temperature power. Only black bodies, which are hypothetical surfaces that accumulate heat from all occurrences, are subject to the rule.








