Table of Contents
Introduction
Raoult’s law was designated after François-Marie Raoult, a French chemist who discovered while conducting an experiment that when substances were mixed in a solution, the vapor pressure of the solution decreased simultaneously. Raoult’s law, which was established in 1887, is also known as the law of thermodynamics. Raoult’s law requires ideal solutions. It can be said that “perfect solutions have the same intermolecular interactions as pure components and the same thermodynamic blending properties as perfect different gases.” There are numerous options for other concepts in Chemistry; however, this law applies only to ideal solutions. However, it still works well for dilute solutions that are insolvent. In reality, the calculation of Raoult’s law for the extremely dilute solution will be greater as pressure decreases.
Raoult’s law is broadly used to calculate the contribution of individual liquid or solid mixture components to the total pressure exerted by the system. It is used to quantify the decrease in vapor pressure of a non-volatile solute. As we know, it is mostly applicable to ideal solutions, i.e. no change in enthalpy or non-mixing volume. A liquid bonding strength can also be quantified. When the concentration is increased by adding solute, the solute-solvent interaction becomes critical.
Overview
Everyone knows that the ideal gas law is similar. The only exception to this rule is that it only applies to ideal solutions. We’ve all read the ideal gas law, and we know that it is based on the assumption that the intermolecular forces present between different molecules are zero or non-existent. We assume that the existing intermolecular forces between similar and dissimilar molecules are equal in Raoult’s Law. It can also be applied to less-than-ideal solutions. This is accomplished, however, through the incorporation of several factors in which the interactions of various substances are considered.
Colligative properties are a concept or process. According to the reviews, the additional solute will fill the gaps between solvent particles to take up space while adding a solute with a lower vapor pressure to it. This lowers vapor pressure because less solvent is able to break free and enter the gas phase, remaining on the solvent’s surface. Raoult’s Principle begins with a simple visual approach and progresses to a more thorough one based on entropy.
The number of particles adhering to the surface is the same as an equilibrium, in this case, the set-up of the number of particles breaking away from the surface. Keep in mind that saturated vapor pressure is what you get when a liquid is sealed in a container. A certain ratio of solvent molecules will have enough energy to escape from the surface (e.g., 1 in 1000 or 1 in a million). If you reduce the number of solvent molecules on the surface, you will reduce the number of molecules that can run away at any given time. The ability of molecules will not be affected if the vapor adheres to the surface again.
If the vapor comes into contact with a solute-covered portion of the interface, it may cling to a solvent molecule. If there is no obvious attraction between the solvent and the solute, there would be no solution in the first place.
There will be fewer liquid particles in the vapor. As a result, once an equilibrium is reached, it is unlikely that they will fail. Nothing beats returning problems to them. If there are fewer particles in the vapor at parity, the saturated vapor pressure is lower.
Raoult’s law
Raoult’s law dictates that the partial vapor pressure of a solvent in a solution (or mixture) is equal to or identical to the pure solvent’s vapour pressure multiplied by its mole fraction in the solution.
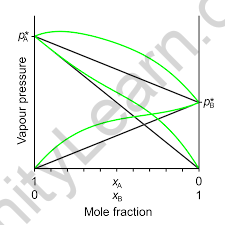
There will be deviations from Raoult’s law if there are adhesive or cohesive forces between two liquids.
When the vapor pressure is lower than expected by the law, this is referred to as a negative deviation. This happens when the forces between particles are stronger than in pure liquids. This behavior can be seen in a mixture of chloroform and acetone, for example.
The deviation is caused by hydrogen bonds in this case. Another example of a negative deviation is a solution of hydrochloric acid and water.
When the cohesion of similar molecules exceeds the adhesion of unlike molecules, there is a positive deviation. As a result, the vapor pressure is higher than expected. Both components of the mixture are more likely to escape solution than if the components were pure. This behavior is observed in benzene-methanol mixtures as well as chloroform-ethanol mixtures.
Raoult’s law formula
Raoult’s law can be expressed as,
Psolution = ΧsolventP0solvent
Here,
Psolution = vapor pressure of the solution
Χsolvent = mole fraction of the solvent
P0solvent = vapor pressure of the pure solvent
When more than one solute is added to the solution, the component of each solvent is added to the total pressure.
Raoult’s law is similar to the ideal gas law, except that it applies to solution properties. The ideal gas law assumes perfect behavior, in which intermolecular forces between dissimilar molecules equal forces between similar molecules. Raoult’s law is based on the assumption that the physical properties of the components of a chemical solution are identical.
The information about Raoult’s law from various chemistry-related articles is available here. Raoult’s law dictates that the partial vapor pressure of a solvent in a solution (or mixture) is equal to or identical to the pure solvent’s vapor pressure multiplied by its mole fraction in the solution. Students who want to flourish in chemistry need to be well known about this law to get deep knowledge about it to do well on their exams.
The definition, detailed explanation, and formula are provided here to assist students in effectively understanding the respective topic. Continue to visit our website for additional chemistry help.
FAQ’s
What is an example of Raoult's Law?
Assume we have 100 mL of water and 100 mL of ethylene glycol in two separate containers. If the vapor pressure of pure water is 500 mmHg, we want to calculate the vapour pressure of the solution formed by combining the two substances.
What are the applications of Raoult’s law?
Raoult's law is broadly used to calculate the contribution of individual liquid or solid mixture components to the total pressure exerted by the system. It is used to quantify the decrease in vapor pressure of a non-volatile solute.
Can we apply Raoult’s law to all kinds of solutions?
Raoult's law only applies to ideal solutions, not all solutions. The solvent-solute interactions in an ideal solution are the same as the solvent-solvent or solute-solute interactions. This implies that in their pure states, solutes and solvents both require energy to escape to the vapor phase. Above all, the ideal solution is the only one that applies to all concentrations. If the total number of solute particles changes in an association, it is due to association and dissociation.



