Table of Contents
Conduction Band: When electrons are energized, they can bounce up into the conduction band, which comprises electron orbitals. When electrons are in these orbitals, they have enough energy to move freely through the material. An electric current flows as a result of the movement of electrons. The valence band is the most distant electron orbital of a particle of a specific material in which electrons are involved.
The bandgap is the energy difference between the valence band’s highest occupied energy state and the conduction band’s least abandoned condition, indicating a material’s electrical conductivity. A large bandgap indicates that a large amount of energy is required to energize valence electrons to the conduction band. When the valence and conduction bands overlap, as in metals, electrons can quickly bounce between the two groups, indicating that the material is extremely conductive.
When an electric field is applied to quicken them, or the temperature of the crystal is raised, the electrons in this energy band can expand their energies by moving to higher energy levels within the band. Conduction electrons are the name given to these electrons.
The conduction band is a band of electron orbitals in which electrons can jump from a lower energy level to a higher energy level when excited. Electrons have enough energy to move freely through a material when they are excited.
There is an electric current flow due to this flow of electrons. The energy gap between the valence band’s highest occupied energy state and the conduction band’s lowest energy state is known as the bandgap, which is used to determine the material’s electrical conductivity. This large gap represents how much energy is required to excite electrons to the conduction band.
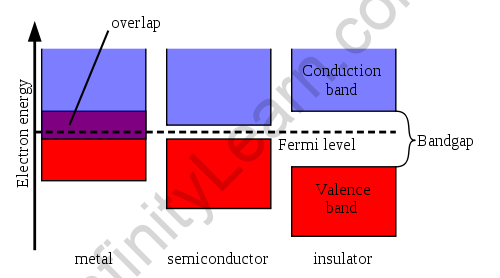
Conduction Bands in Semiconductors and Metallic Conductors
The conduction band and the valence band in metallic conductors overlap. At sufficiently low temperatures, the conduction band in semiconductors and inductors is devoid of electrons. The thermal excitation of electrons produces conduction electrons in a lower energy band or by impurity atoms in the crystal.
Forbidden Band
The forbidden band is the energy difference between a conduction and valence band. Some of its characteristics are as follows:
There are no free electrons.
There is no energy.
The nature of the substance determines the size of the forbidden energy gap. The forbidden energy gap decreases very slightly as temperature rises.
FAQs
What exactly is a conduction band?
Conduction Band: Because the outermost electrons are not tightly bound to the nucleus, they can leave the outermost orbit at room temperature and become free electrons. These free electrons tend to conduct current in conductors, which is why they are called conduction electrons. As a result, the conduction band is the band with the lowest occupied energy and contains conduction electrons. A large bandgap reveals the conditions required to energize valence electrons to conduction bands. When a metal's valence and conduction bands overlap, electrons can quickly bounce between the two groups, indicating that the material is extremely conductive.
What is the significance of the term conduction band?
Band of Conduction: Because the outermost electrons are not tightly bound to the nucleus, they can leave the outermost orbit at room temperature and become free electrons. These free electrons tend to conduct current in conductors, which is why they are called conduction electrons.







