Table of Contents
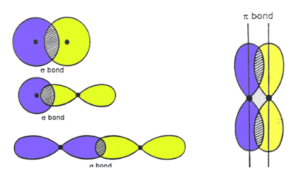
The overlapping of atomic orbitals distinguishes sigma and pi bonds from other covalent bonds. The overlapping of atomic orbitals causes covalent bonds to form. Sigma bonds are created when two atomic orbitals overlap head-to-head, whereas pi bonds are generated when two atomic orbitals overlap laterally.
The manner in which atomic orbitals overlap determines the bond length, bond angle, and bond enthalpy. This overlap happens in two ways, resulting in two types of covalent bonds: sigma and pi.
The Sigma Bond is a type of bond that is formed when two people come together
Positive (same phase) head-on overlap of atomic orbitals along with the internuclear axis forms this sort of covalent connection. Due to the direct overlapping of the participating orbitals, sigma bonds are the most powerful covalent bonds. Electrons are the electrons that participate in a bond.
Sigma bonds are the most common type of single bond. The combinations of atomic orbitals below can be used to make them.
S-S Overlapping is a term used to describe when two or more objects overlap
One of each participating atom’s orbitals is overlapping head-on along the internuclear axis in this type of overlapping. Before overlapping with another s orbital, it must be half-filled.
Above is an illustration of a sigma bond formed by the overlap of two s orbitals. Each hydrogen atom has a half-filled s orbital, which causes this form of overlap in H2 molecules.
Overlapping (S-P) is a term used to describe when two or more
Along the internuclear axis, a covalent bond is formed when one half-filled s orbital overlaps with one half-filled p orbital. T
Ammonia is a good example of this type of overlapping. The overlap of the nitrogen atom’s 2px, 2py, and 2pz orbitals with the 1s orbitals of the three hydrogen atoms forms three sigma bonds in an NH3 molecule.
Overlap between P and P
Each participating atom’s half-filled p orbital overlaps head-on along the internuclear axis under this circumstance.
The 3pz orbitals of two chlorine atoms in a Cl2 molecule are p-p overlapping. It’s worth noting that a sigma bond is formed when two p orbitals overlap head-to-head, whereas pi bonds are formed when the orbitals overlap laterally.
The Pi () Bond is a mathematical concept that describes the relationship between two numbers.
Pi bonds are generated when atomic orbitals intersect in a sideways positive (same phase) direction perpendicular to the internuclear axis. The atomic orbitals’ axes are parallel to one other during bond formation, but the overlapping is perpendicular to the internuclear axis. Because of the substantially smaller degree of overlapping, pi bonds are often weaker than sigma bonds. A conventional triple bond is made up of two bonds and one sigma bond, whereas a double bond is made up of one sigma bond and one pi bond. It’s worth noting that a sigma and pi bond combination is always more powerful than a single sigma bond.
Difference between a Sigma and a Pi Bond?
Below is a list of the key differences between sigma and pi bonds.
| Sigma Bond | Pi Bond |
| The orbitals that overlap can be pure or hybrid. | The orbitals that overlap must be unhybridized. |
| These relationships are strong and have a lot of energy attached to them. | These ties aren’t very strong. |
| Can subsist on its own. | A sigma connection must also be present. |
| Has an effect on the molecule’s form | Has no influence on the shape of molecules. |
FAQs
In a double or triple bond, how many Pi bonds are there?
Two pi bonds and one sigma bond combine to form a triple bond. One sigma and one pi bond make up a double bond. All sigma bonds are single bonds.
Q. What Orbital Combinations in Sigma Bonds are Possible?
Ans: Sigma bonds are formed by the following three types of overlap conditions:
- overlapping s-s
- overlapping s and p
- overlapping p and p










