Table of Contents
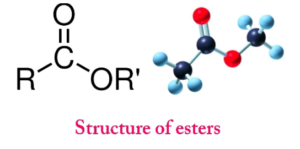
Esters are a class of chemical compounds generated by connecting an alcohol group with a group of organic acids while losing water molecules. Esters are polar, but not as much as alcohols. These chemical substances contribute to the production of hydrogen bonds as hydrogen-bond acceptors, but they cannot produce hydrogen bonds as donors. Some water solubility is aided by this ability to participate in hydrogen bonding.
Carboxylic acids and alcohols are used to make these esters. In the presence of a catalyst, usually concentrated sulphuric acid, alcohol, and carboxylic acid are heated. This is a type of condensation reaction in which two or more molecules combine to generate a larger molecule known as an ester and a smaller molecule, usually water.
Structure and Bonding
The carbonyl center in esters causes the C–C–O and O–C–O angles to be 120 degrees. Because rotation around the C–O–C bonds has a low barrier, esters, unlike amides, are structurally flexible functional groups. Physical qualities reflect their flexibility and low polarity; they are less stiff (lower melting point) and volatile (lower boiling point) than amides. Alpha-hydrogens on esters have a pKa of about 25.
Because of a combination of hyperconjugation and dipole minimization effects, many esters have the potential for structural isomerism, but they prefer to adopt the s-cis (or Z) conformation over the s-trans (or E) conformation. The type of the substituents and, if present, the solvent can impact the predilection for the Z conformation. Due to their cyclic nature, lactones with short rings are confined to the s-trans (i.e. E) conformation.
Characterization and physical qualities
Esters have a lower polarity than alcohols, but they have a higher polarity than ethers. Unlike their parent alcohols, they can participate in hydrogen bonding as hydrogen-bond acceptors but not as hydrogen-bond donors. Water solubility is conferred via the ability to participate in hydrogen bonding. Esters do not self-associate because they lack the ability to donate hydrogen bonds. As a result, esters have higher volatility than carboxylic acids of comparable molecular weight.
Instances and Applications
Esters are found all over the world and are frequently employed in the industry. Fats are generally triesters derived from glycerol and fatty acids in nature. [10] The aroma of many fruits, including apples, durians, pears, bananas, pineapples, and strawberries, is due to esters. [11] Several billion kilograms of polyesters are produced industrially each year, with polyethene terephthalate, acrylate esters, and cellulose acetate being the most prominent products.
Uses of Esters:
The saponification process comprises the hydrolysis of fats or oils (triglyceride ester) in the presence of strong alkalies, such as (NaOH) or (KOH), to produce glycerol and soap (sodium or potassium salt of fatty acid).
Polyesters are polymers formed by the condensation of two monomers: dibasic acid and dihydric alcohol. The most common polyester is Dacron fibres, which are made from the interaction of terephthalic acid and ethylene glycol (ester formation reaction).
The ester’s alcohol end combines with the carboxylic group of a new acid molecule, or the acid end of the molecule may be linked by a new alcohol molecule, and the condensation process continues.
Polymers of esters (polyester): Esters as pharmaceuticals: Organic esters are used in the production of many drugs, including aspirin and Marookh oil. Aspirin is the most common and simplest, while Marookh oil is a local oil applied to the skin to relieve rheumatism pains. The acid used in the production of these two drugs is salicylic acid, which has both carboxylic and hydroxyl groups and reacts as an acid or an alcohol.
Physical properties of Esters:
Points of evaporation
With the same number of carbon atoms, small esters have boiling temperatures similar to aldehydes and ketones. Esters are polar compounds, like aldehydes and ketones, with dipole-dipole interactions and van der Waals dispersion forces. Because they don’t establish ester-ester hydrogen bonds, their boiling temperatures are much lower than acid with the same amount of carbon atoms.
In water, solubility
Small esters are water-soluble, however, their solubility diminishes as the chain length increases, as seen below:
Although esters cannot hydrogen bond with one other, they may hydrogen bond with water molecules, which explains the trend of insolubility. A hydrogen bond can be formed when one of the partly positive hydrogen atoms in a water molecule is sufficiently attracted to one of the lone pairs on one of the oxygen atoms in an ester. There are also dipole-dipole attractions and dispersion forces.
The energy required to solvate the ester is partially released by forming these intermolecular interactions. The hydrocarbon portion of the chain forces itself between water molecules as the chain length increases, breaking the relatively strong hydrogen bonds between water molecules without providing an energetic compensation; additionally, the water molecules are forced into an ordered alignment along the chain, reducing the system’s entropy.
Oils and fats
Long-chain, complex esters make up animal and vegetable fats and oils. The physical differences between fat (such as butter) and oil (such as sunflower oil) are due to differences in the melting points of the esters in the mixture. If a substance’s melting point is below room temperature, it is a liquid – an oil.
It will be a solid – a fat – if the melting point is higher than room temperature.
Also read: Alkaline Earth Metals
FAQs:
What happens when an ester is formed?
The condensation process between alcohol and carboxylic acid produces esters. Esterification is the term for this process. Two molecules unite to form a bigger molecule while a smaller molecule is eliminated in a condensation reaction. This tiny molecule is water during esterification.
What are the four applications of esters?
Phosphate esters are used frequently in the industry as solvents, plasticizers, flame retardants, gasoline and oil additives, and pesticides, and are biologically important (nucleic acids are among them). In the production of colours and pharmaceuticals, sulfuric and sulphurous acid esters are utilised.









