Table of Contents
Introduction
Halogenation is a type of chemical reaction in the addition of one or more halogens to a substance or molecule. The stoichiometry and route of halogenation are regulated by the functional groups and structural properties of the organic substrate and the unique halogen.
A brief outline
When an alkane is halogenated, one or more halogen atoms are swapped for hydrogen atoms, resulting in a hydrocarbon derivative. Alkanes are often unreactive chemicals and they are non-polar and lack functional groups where reactions can take place.
Important concepts
The halogenation of alkanes is an example of a substitution reaction, which is a common type of reaction in organic chemistry. A substitution reaction occurs when a small component of a responding molecule replaces an atom or even the atom’s group on a hydrocarbon or even its derivative.
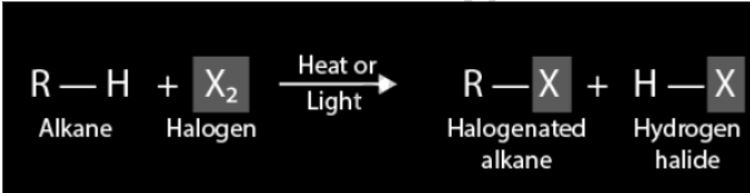
The replacement of a single halogen atom for one of the hydrogen atoms in an alkane has a generic equation.
Qualities of alkane halogenation.
- An alkane is addressed by the image R-H. In the present circumstance, R represents an alkyl bunch. At the point when a hydrogen iota is added to an alkyl bunch, the alkyl gathering’s guardian hydrocarbon is shaped.
- On the item side, the image R-X means the conventional equation for a halogenated alkane. A halogen iota is addressed by the letter X.
- Placing reaction conditions on the equation arrow that divides reactants and products is a good way to keep track of them. The existence of heat or light is required for the halogenation of an alkane.
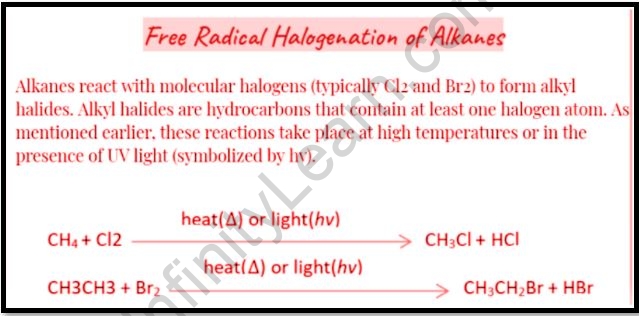
The homolytic breakdown of a Cl-Cl bond to produce two Cl atoms is the first step. The freshly produced methyl radical removes a Cl atom from a chlorine molecule, resulting in chloromethane and the formation of another Cl atom. Chlorine, chloromethane, and ethane are the termination products.
Alkanes can be functionalized using the free-radical halogenation technique. The count of identical C-H bonds, which is prevalent in all but the basic alkanes, is a major constraint of radical halogenation, making selective reactions difficult to execute.
Significance of halogenation of alkanes in IIT JEE exam
Halogenation of alkanes is a type of organic chemistry that studies specific reactions. This topic carries a 6.33 per cent weighting in the IIT JEE exam. All three primary units of chemistry – organic, inorganic, and physical chemistry – were given similar weightage in terms of high weightage chapters for JEE.
FAQs
Halogenation is a chemical process that occurs when one or more halogens are added to a material.
The reaction of a halogen with an alkane in the presence of ultraviolet (UV) light or heat results in the creation of a haloalkane (alkyl halide).
Alkanes are subjected to a limited number of reactions. Combustion and halogenation are the two reactions that further imports undergo What is the process of halogenation?
What is the essence of the halogenation of alkanes mechanism?
What happens when alkanes react?









