Table of Contents
Kinetics of Radioactive Decay Chemistry
Radioactive decay (also referred to as nuclear decay, radioactivity, radioactive disintegration, or nuclear disintegration) is the process by which an unstable atomic nucleus loses energy through radiation. Radioactive material is one that contains unstable nuclei. Alpha decay (-decay), beta decay (-decay), and gamma decay (-decay) are three of the most common types of decay, all of which involve the emission of one or more particles.
The weak force is in charge of beta decay, while the electromagnetic and strong forces are in charge of the other two. At the atomic level, radioactive decay is a stochastic (i.e. random) process. It is impossible to predict when an atom will decay, according to quantum theory, regardless of how long the atom has existed. For a large number of identical atoms, the overall decay rate can be expressed as a decay constant or as a half-life.
The half-lives of radioactive atoms range from nearly instantaneous to far longer than the age of the universe. The parent radionuclide (or parent radioisotope is the decaying nucleus, and the process yields at least one daughter nuclide. With the exception of gamma decay or internal conversion from a nuclear excited state, the decay is a nuclear transmutation that results in a daughter with a different number of protons or neutrons (or both). When the number of protons in an atom changes, a new chemical element is formed.
When the nucleus ejects an alpha particle, this causes alpha decay (helium nucleus). Beta-decay occurs in two ways: beta-minus decay, in which the nucleus emits an electron and an antineutrino in a process that converts a neutron to a proton, and beta-plus decay, in which the nucleus emits an electron and an antineutrino in a process that converts a neutron to a proton. When the nucleus emits a positron and a neutrino in a process that converts a proton to a neutron, this is referred to as beta-plus decay.
Gamma decay begins with the emission of an alpha or beta particle from a radioactive nucleus. The resulting daughter nucleus is typically left in an excited state, but it can decay to a lower energy state by emitting a gamma-ray photon.
Extremely neutron-rich nuclei formed by other types of decay or after many successive neutron captures occasionally lose energy via neutron emission, resulting in a change from one isotope of the same element to another. In the process of electron capture, the nucleus may capture an orbiting electron, causing a proton to convert into a neutron.
Following that, a neutrino and a gamma-ray are emitted. A nucleus heavier than an alpha particle is emitted during cluster decay and nuclear fission.
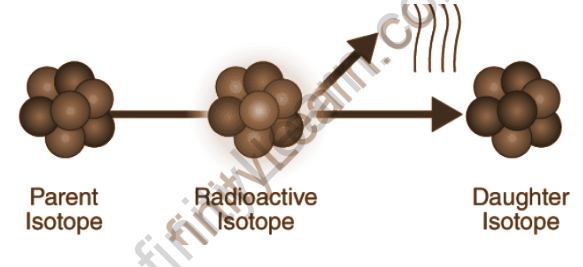
Overview
First-order kinetics governs the decay of radioactive nuclei. As a result, the rate laws for first-order reactions can be used to derive the half-life equation and integrated rate law for radioactive decay processes. The resulting equations can be used to calculate the rate constant k of a decay process and the amount of radioactive isotope left after a given time period. Many significant things occur around us that go unnoticed, such as the atom’s emission and absorption phenomena. Radioactivity is a property of matter that causes the spontaneous release of energetic subatomic particles. Because radioactive decay is a random process, it is impossible to predict which atom from a collection of atoms will disintegrate at a given time. As a result, the average amount of radionuclides dissolving over time can only be mentioned.
This is the rate at which a radionuclide breaks down. Radioactivity is a nuclear reaction that occurs when an unstable nucleus decays. The decay of the more unstable nucleus is referred to as radioactivity. The two forces, namely the nucleus’ enormous powers of attraction and repulsion, collaborate to keep the nucleus together.
Because of the presence of an unstable nucleus in the element’s radioisotope, the atom particles cannot be bounded. The isotopes are constantly decaying in order to stabilize themselves, releasing a large amount of energy in the form of radiation. Transmutation is the process of converting an isotope into an element of a stable nucleus. It can occur both naturally and artificially.
Properties of Radioactive Decay
Radiation is a type of energy that travels through space. It is emitted by one thing and absorbed by another. The speed at which they move is determined by their kinetic energy. Particulate radiation can be emitted by radioactive decay, cosmic rays, nuclear processes, and other sources. A radioactive element is undergoing a spontaneous transition. The transformation theory made no mention of a specific mechanism within the atom that would cause the release of alpha particles at a specific time, resulting in a chemical change.
Calculations Using the First Order Rate Equation: r = k[N]
Because the rate of radioactive decay is first order, we can write: r = k[N]1, where r is the rate of decay measurement, k is the isotope’s first order rate constant, and N is the amount of radioisotope at the time the rate is measured. The rate of decay is often referred to as the isotope’s activity and is measured in Curies (Ci), with one curie equaling 3.700 x 1010 atoms decaying per second. The rate constant can be calculated using the amount of radioisotope and the activity of the sample.
Calculations Using the First-Order Rate Equation: ln(N/No) = -kt
Using the first-order kinetics equation, the following equation can be derived:
ln(N/No) = -kt
where “N” denotes the amount of radioisotope left after time “t” has passed. “No” represents the initial amount of radioisotope at the start of the period, and “k” represents the rate constant for the radioisotope under study. The units of measure for N and No in this equation can be grammes, atoms, or moles. It doesn’t matter as long as they’re comparable measurements. The units of time are determined by the unit of measure for the rate constant. The “N/No” ratio represents the percentage of activity compared to the activity at time zero. This equation can be used in a variety of ways.
Types of Radioactive Decay
The nucleus decays into one of three types of nuclear decay:
During alpha decay, the helium nucleus is emitted.
During beta decay, electrons are emitted.
The gamma decay produces high-energy photons.
- Particles of Alpha
The nucleus of a helium atom is made up of two neutrons and two positively charged protons. Some radioactive nuclei emit alpha particles. When alpha particles pass through tissue, they deposit more energy than gamma or beta particles. A thin layer of light material, such as a sheet of paper, can prevent alpha particles from penetrating the skin’s outer, dead layer. As a result, they do not harm living tissue when they are outside the body. When inhaled or eaten, however, alpha-emitting atoms are especially dangerous because they deliver massive amounts of ionising energy to living cells. Other terms for the same thing include beta particle, gamma-ray, neutron, and x-ray.
- Particles of Beta
This is an important aspect of radioactive decay in which electrons are discharged from the nucleus of a decaying atom. Even if they are blocked by a thin sheet of aluminium, beta particles can penetrate the dead skin layer and cause burns. Depending on the amount of radiation received, they can be fatal, and they can pose a significant direct or external radiation threat. If beta-emitting atoms are swallowed or inhaled, they pose a significant internal radiation risk.
- Gamma Rays
When the nuclei of certain radionuclides transition from a higher to a lower energy state, high-energy electromagnetic radiation is produced. These rays have a short wavelength and a large amount of energy. Because the energy of all gamma rays emitted by a particular isotope is the same, scientists can determine which gamma emitters are present in a sample. Gamma rays penetrate tissue more deeply than beta or alpha particles, but they leave fewer ions in their wake, which may cause cell damage. The properties of X-rays and gamma rays are very similar.
FAQ’s:
Q. What exactly is kinetic radioactive decay?
Ans: Because the rate of radioactive decay is first order, we can write: r = k[N]1, where r is the rate of decay measurement, k is the isotope’s first order rate constant, and N is the amount of radioisotope at the time the rate is measured.
Q. What is the reaction order of radioactive disintegration?
Ans: The radioactive disintegration reactions are of the first order because the rate of disintegration is determined solely by the concentration of radioactive material.
For more visit Important kinetic theory of gas formulas for JEE



