Table of Contents
The Sandmeyer reaction has been thought to be an excellent example of a radical-nucleophilic aromatic substitution. One such reaction seems to be a useful tool for replacing an amino group on an aromatic ring with different substituents. The Sandmeyer reaction converts an amino group attached to an aromatic ring into a diazonium salt that can be converted into various functional groups.
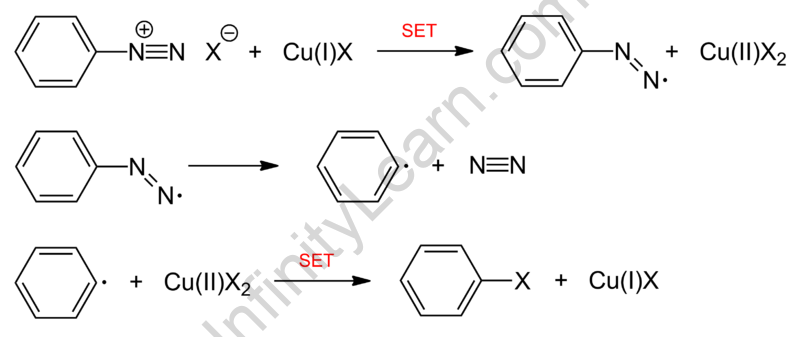
The Sandmeyer reaction has been governed by a free radical mechanism. In fact, the formation of aryl halides from primary arylamines is a two-step process that involves the formation of diazonium salts and the transformation of diazo intermediates into aryl halides (displacement with a nucleophile). Surprisingly, the nucleophile can be a halide anion, cyanide, water, or other substance.
To elaborate further, the Sandmeyer reaction mechanism begins with a single electron transfer from the copper to diazonium. It thus produces a non-participating diazo radical as well as copper(II) halide. The diazo radical afterward emit a molecule of nitrogen gas, forming an aryl radical that reacts with the copper(II) halide to restore the catalyst [copper(I) halide]. As a consequence of all of this, the final product, aryl halide, is acquired.
Formation of Nitrosonium Ion
In the test, sodium nitrite and acid are combined to form nitrous acid. Following that are two protonation steps in which one equivalent of water is removed. As a result, the nitrosonium ion has been produced (nitrogen monoxide cation).
So when the ion is further reacted with an aromatic or heterocyclic amine, it becomes an electrophile (for e.g. aniline). The diazonium salt has been finally formed. The reaction takes place at temperatures ranging from 25 to 30oC degrees Celsius.
Importance of Sandmeyer Reaction
- While studying aromatic chemistry, the Sandmeyer reaction is crucial. The reaction can be used to create substitution patterns that are not always possible with direct substitution.
- A principal arylamine is primarily diazotized in the reaction to produce an aryl diazonium salt, which is then reacted with a halide ion to produce the desired aryl halide product.
- This is based on an electron-transfer mechanism that includes free radicals.
- The Sandmeyer reaction does have a wide range of synthetic applications and is useful in conjunction with electrophilic aromatic substitution.
Applications of Sandmeyer Reaction
- It is being used in the hydroxylation process to convert aryl amines to phenols, which results in the formation of an aryl diazonium salt.
- To produce aryl compounds during the trifluoromethylation process. The compounds have distinct chemical properties that have a wide range of practical applications.
- The Sandmeyer reaction is being used in the cyanation process to produce benzonitriles. The reaction is often discovered during the synthesis of neoamphimedine, a chemical compound used as an anti-cancer drug that targets topoisomerase II.
- To produce aryl halides. A few of the solvents used in this process include diiodomethane for the synthesis of aryl iodides, bromoform for the synthesis of aryl bromides, and chloroform for the synthesis of aryl chlorides.
FAQs
What type of reaction is Sandmeyer's reaction?
The substitution reaction has been widely used in the production or synthesis of aryl halides from aryl diazonium salts.
What is the key difference between the Sandmeyer reaction and the Gattermann reaction?
The type of reactant utilised has been one of the key differences between the Sandmeyer and Gattermann reactions.







