Table of Contents
Introduction
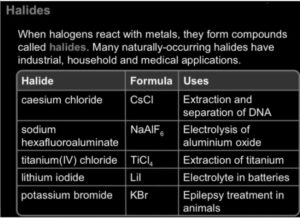
Halide definition: To form a fluoride, chloride, or potentially Tennessee compound, a halide is a binary phase in which one part is a halogen atom and another part is an element or radical which is less electronegative than the halogen. Under the right conditions, alkali metals react directly with halogens to form halides with the general formula MX (X = F, Cl, Br, or I). Many salts are halides, as seen by the hal- syllable in halide and halite. At room temperature, all Group 1 metals create halides, which are white solids. A halide ion is a negative charges halogen molecule. Fluoride (F), chlorine (Cl), bromide (Br), iodide (I), and astatide (At) are really the halide anions. All ionic halide salts contain these ions.
A brief outline
Halides are found in halide minerals. All of these halides are colorless, crystalline solids with large negative formation enthalpies. The halide ion is defined as a halogen atom with a negative charge. The halide anion is contained in halide minerals. 2 major halide minerals include fluorite and halite. The main constituent of hydrogen fluoride is fluorite. The mineral halite is a dominant contributor to sodium chloride. Bischofite is a key magnesium source. The coastal evaporite deposits contain many halides. Iodide, bromide, chloride, and fluoride are some of the halogen anions.
Silver nitrate solution is used to test various halide compounds. Kl, KBr, and KCl are a few examples. Precipitation is created when halogen combines with silver nitrate solution, and the color varies based on the type of halides. Silver Fluoride with no precipitate is one of them. The precipitate of silver bromide with a pale-yellow color. Green precipitate of silver iodide. A white precipitate of silver chloride.
Important concepts
- Aryl halide
Aryl halides are found all over nature, although they are most typically created by marine creatures that use the chloride and bromide found in ocean waters. There are also several chlorinated and brominated aromatic chemicals, such as tyrosine, tryptophan, and other pyrrole derivatives. A few of these found naturally aryl halides have a medical use.
The unique bonding of a halogen straight to a benzene ring distinguishes an aryl halide. One of the most essential structural considerations is the trend in bond lengths among the four aryl halides. Because the trend in atomic size is F < Cl < Br < I (meaning fluorine is smaller than chlorine, which is smaller than bromine, etc.) Because we’re dealing with chemical bonding on a minuscule size, the bond lengths are quantified in picometers, which is a minuscule unit of measurement. This is because when the halogen’s size increases, the connection must lengthen to accommodate the larger atom attached to the benzene ring.
Electrophilic aromatic substitution, or EAS for short, occurs easily with aryl halides. Because of the halogen’s electronegative nature, when another atom or group of atoms is joined to the benzene ring, they invariably go to the ortho and para positions. This is because these sites provide the most stability for the carbocation that will eventually form when a carbon-carbon double bond splits.
- Allylic halide
The structural formula for an allyl group is H2C=CHCH2R, where R represents the rest of the molecule. It is made up of a vinyl group (CH=CH2) and a methylene bridge (CH2). The term Allium sativum comes from the Latin term for garlic. Theodor Wertheim identified and called “Schwefelallyl” an allyl derivative from garlic oil in 1844. The name allyl refers to a variety of compounds related to H2C=CHCH2, some of which are useful or commonplace, such as allyl chloride.
The allylic position, also known as the allylic site, is a location close to an unsaturated carbon atom. A group associated with this location is frequently referred to be allylic. As a result, CH2=CHCH2OH “possesses an allylic hydroxyl group.” The CH bonds in allylic carbon centers are about 15% weaker than the CH bonds in typical sp3 carbon centers, making them more reactive. This increased reactivity has a number of practical implications.
- Vinyl halide
A vinyl halide is a chemical having the formula CH2=CHX (X = halide) in organic chemistry. Any alkenyl group is commonly referred to as vinyl. Because of this, alkenyl halides having the formula RCH=CHX are also known as vinyl halides. Vinyl chloride, which is manufactured in millions of tons per year as a precursor to polyvinyl chloride, is the most important member of this class in terms of applications. Another commercial product is polyvinyl fluoride. Vinylidene chloride and vinylidene fluoride are related chemicals.
Grignard reagents and associated organolithium reagents are made up of vinyl bromide and similar alkenyl halides. Base elimination produces the equivalent alkyne from alkenyl halides. Their usage in cross-coupling reactions is particularly crucial.
Significance of halides in NEET exam
According to the paper reviewed in the previous year’s NEET chemistry papers, the topic was assessed as easy by the majority of test-takerstakers. Candidates must prepare for three components of the NEET exam: Organic, Inorganic, and Physical Chemistry, in order to achieve a high score. General Organic Chemistry, Halides, Amines, and the Periodic Table can all be used to ask 20 to 25 questions. On the basis of a chemistry paper, candidates must mark a total of 45 questions in NEET.
Also read: Important Topic of Chemistry: Oxoacids
FAQs
Q. What are the applications of alkyl halide?
Ans: Many halogen-containing chemical compounds can be found in nature, and some of them are clinically beneficial.
These types of chemicals have a wide range of applications in both business and everyday life. They are utilized as starting materials for the synthesis of a wide range of organic molecules and as solvents for comparatively non-polar chemicals. Chloramphenicol, a chlorine-containing antibiotic generated by soil bacteria, is particularly beneficial in the treatment of typhoid fever.
A nucleophile reacts with a haloalkane that has a partial positive charge on the halogen-bonded carbon atom. A substitution process occurs, and the halogen atom known as the leaving group is released as a halide ion. The substitution reaction is known as a nucleophilic substitution reaction because it is triggered by a nucleophile. In halides, what is the nucleophilic substitution reaction?
Q. What are the many ways to make alkyl and aryl halides?
Ans: Numerous ways exist for producing haloalkanes and haloarenes from other chemical molecules. The following are some techniques for making alkyl halides and aryl halides:
- Alkyl Halide Preparation from Alkenes
- Alkyl Halide Preparation from Alcohols
- Free Radical Halogenation for the Production of Alkyl Halides
- Electrophilic Substitution Reactions for the Production of Aryl Halides
- Sandmeyer’s Reaction is used to make aryl halides.







