Table of Contents
Valence is a concept used to quantify covalent bonding between atoms in molecules rather than ionic bonding. The driving force for a quantitative fixation in a convenient valence index was chemical reactivity. It is fascinating that the first manifestation of a valence theory began with a consideration of electronic distribution in condensed unsaturated hydrocarbons. Positions with greater or lesser reactivity were thought to appear in the molecule if the electrons were more unevenly distributed than in benzene, as in anthracene. Svartholm also recognized that these molecules have two competing types of reactivity, namely reactivity at atoms and reactivity at bonds. His analysis only allowed him to compare different regions in a single molecule, not several molecules.
It took several years for this simple picture to be improved from both sides. Daudel and Pullman normalized the portions of electrons attached to bonds and nonbonding electrons, providing reactive sites at atoms so that the sum of all portions equaled the number of electrons. This simple but effective procedure allowed for the comparison of different molecules and revealed trends in reactivity ranging from benzene to anthracene. According to this analysis, the position in naphthalene is more reactive than the carbon positions in benzene. This was the intuitive birth of the concept of free valence for various molecular sites and molecular systems.
In chemistry, an element’s valence or valency is the measure of its ability to combine with other atoms to form chemical compounds or molecules. Following significant advancements in the field of electronic theory of periodicity of elements, the valence theory was reformulated and described, incorporating electronic structures and interatomic forces. As a result, several new concepts such as ionic valence, covalence, oxidation number, coordination number, and metallic valence were introduced, which also explain the various modes of atomic interactions.
It is critical to understand the valencies of the common elements. This will provide you with a fundamental understanding of valency. Try to identify the valency of various elements. This will provide you with a better understanding of how valency works. By understanding valency, you can better predict how chemicals will react with one another. This can aid in the development of new compounds for a variety of applications.
Overview
Valence is typically defined as the number of electrons required to fill an atom’s outermost shell. Because there are exceptions, the more general definition of valence is the number of electrons with which a given atom generally bonds or the number of bonds formed by an atom. The maximum number of univalent atoms that can combine with an atom, according to the IUPAC formal definition. The definition is usually based on the maximum number of hydrogen or chlorine atoms. It is worth noting that the IUPAC only defines a single valence value (the maximum), whereas atoms are known to be capable of displaying multiple valences. Copper, for example, typically has a valence of 1 or 2.
A valence is a whole number (positive or negative) that represents one element’s ability to combine with another. In general, the valence number represents the number of electrons in an atom or combined group of atoms that can readily be given up or accepted in order to react with or bond to another atom or group of atoms to form a molecule. When considering an atom’s electronic configuration, electrons are classified into two types based on their positions within the atom: valence electrons and core electrons. Valence electrons are those that occupy an atom’s outermost shell or highest energy level, whereas core electrons are those that occupy the atom’s innermost shell or lowest energy level. In general, valence electrons can participate in chemical bonding formation, but core electrons cannot. The number of valence electrons in an atom is critical to its chemical properties.
Valence
The International Union of Pure and Applied Chemistry (IUPAC) defines the majority of the definitions in Chemistry. As a result, the definitions in valence chemistry are also provided by IUPAC. The IUPAC defines valence meaning as the maximum number of single valence atoms that can potentially merge with an atom of the element understudy, or with a fragment, or for which an atom of this element can be substituted. In modern times, valence is defined as the number of hydrogen atoms that can merge with an element in a binary hydride or twice the number of oxygen atoms that can combine with an element in its oxide or oxides forms.
This definition differs from the IUPAC definition in that an element can have more than one valence. Another modern valence definition states that the valence of a specific atom present in a molecule is the number of electrons that an atom uses in the formation of a bond. This is represented by the formulas for calculating valence electrons, which can also be used to define valence, which is as follows:
Valence = number of electrons in the last/valence shell of a free atom – number of electrons on an atom in unbonded molecules. As a result, the formula for valency definition in chemistry can be shown as
Valence or Valency = number of bonds formed + atom’s formal charge
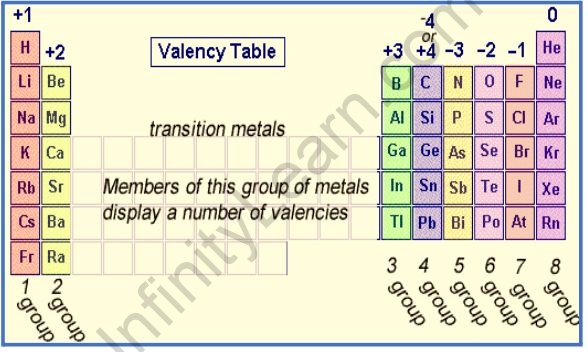
Valence table
The ability of an atom or a group of chemically bonded atoms to form chemical bonds with other atoms or groups of atoms is referred to as valency. The number of outer shells (valence) electrons in an element determines its valency.
An element’s valency is its ability to combine with other elements. Elements in the same periodic table group have the same valency. The valency of an element is proportional to the number of electrons in its outer shell D – if the valencies can be simplified, divide them both by the smaller of the two numbers.
| Number | Element | Valence |
| 1 | Hydrogen | (-1),+1 |
| 2 | Helium | 0 |
| 3 | Lithium | +1 |
| 4 | Beryllium | +2 |
| 5 | Boron | -3,+3 |
| 6 | Carbon | (+2),+4 |
Valence periodic table
A valence electron is an electron that is associated with an atom and can participate in the formation of a chemical bond; for example, in a single covalent bond, both atoms contribute one valence electron to form a shared pair. The presence of valence electrons can affect the element’s chemical properties as well as its ability to bond with other elements: A valence electron can only be in the outermost electron shell of the main group element. The periodic table group (vertical column) in which an element is classified determines the number of valence electrons. Except for groups 3–12 (transition metals), the units digit of the group number identifies how many valence electrons are associated with a neutral atom of an element listed in that column.
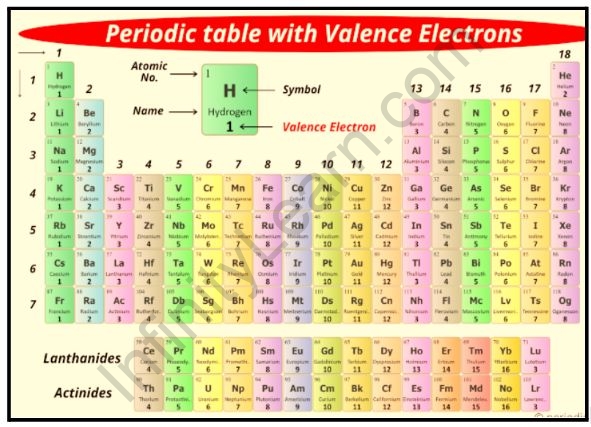
Also read: Important Topics of Chemistry: Valence Electrons
FAQs
Can valency have a negative value?
There is another loss of charge on the atom with the loss and gain of an electron. The charge's loss and gain can have a positive or negative representation, but not valency. The valency electron, or the last unpaired electron in an atomic orbit, is simply stated.
What is an example of valence?
The ability of elements or atoms to combine to form molecules is referred to as valence. When two hydrogen atoms combine with one oxygen atom to form a molecule of water, this is an example of valence.







