Table of Contents
Introduction to Thermal Equilibrium
For the Earth’s temperature to remain constant, it is critical that it maintain thermal equilibrium. The Earth’s incoming solar energy must be balanced, which means it must reflect as much heat as it gets. The greenhouse effect causes the earth to become hotter by slowing heat transport from Earth to space. The natural greenhouse effect is required for the Earth’s temperature to be habitable for people and other animals, and without it, the Earth would be far too cold. However, as carbon dioxide, methane, and nitrogen dioxide levels rise in the atmosphere, the greenhouse effect intensifies, causing somewhat less heat to be released from the Earth than is absorbed in its energy exchanges.
The law of thermodynamics can be applied to the human body. When you’re in a busy space with a lot of people, you start to get hot and sweaty. This is the body’s natural cooling mechanism. Heat is transported from the body to the sweat. Sweat evaporates from your body as it absorbs more heat, becoming much more disorganized and distributing heat to the air, which raises the room’s air temperature. A “closed system” of several sweating persons in a crowded area will rapidly heat the space. This is a perfect example of both the first and second laws of thermodynamics in acts: no heat is lost; instead, it is transferred and approaches equilibrium with the greatest amount of entropy.
| S.NO | CONTENT |
| 1 | INTRODUCTION |
| 2 | BRIEF OUTLINE |
| 3 | IMPORTANT CONCEPTS |
| 4 | THERMAL CONTACT |
| 5 | SIGNIFICANCE |
| 6 | FAQ’S |
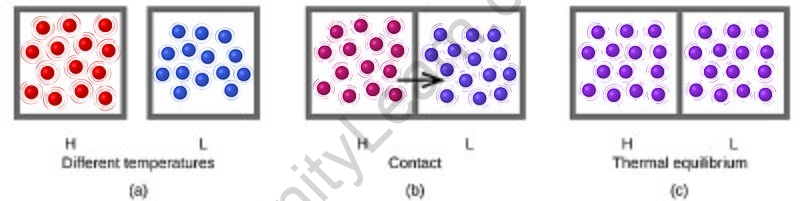
What is Thermal equilibrium?
- The movement of energy from a high to a low temperature is known as heat. The system (or combination of systems) is indeed in thermal equilibrium when the temperatures balance out and the heat stops flowing.
- There is no matter going into and out of the system when it is in thermal equilibrium. Thermal equilibrium is used in the zeroth law of thermodynamics to define how two dissimilar systems can be claimed to be at the same temperature.
- When molten rock rises from a volcano, for example, it radiates heat to the environment until the rock, and the environment reaches the same temperature. Despite the fact that the two systems (rock and air) are dramatically different, thermal equilibrium enables temperature specification in both.
- Thermodynamically balanced systems are always thermally balanced, whereas the opposite is not necessarily true.
- The two systems might achieve thermal equilibrium despite approaching thermodynamic equilibrium if the connection between them allows the transfer of energy as a ‘shift in internal energy’ but not a transfer of matter or energy as work.
Types of thermal equilibrium
-
Relationship between two thermally related things in terms of thermal equilibrium
Thermal equilibrium is an example of equilibrium between two bodies, referring to the transmission through a partially permeable partitioning of matter or work; it is referred to as a diathermal link. The basic meaning of the thermal equilibrium connection, according to Lieb and Yngvason, is that it is reflexive and symmetric. Whether it is transitive or not was included in the core definition. They posit a significant physical axiom, which they name the “zeroth law of thermodynamics,” that thermal equilibrium is a transitive relation, after exploring the semantics of the statement. They say that the recognized similarity classes of systems are referred to as isotherms.
-
An isolated body’s internal thermal equilibrium
The thermal equilibrium of a body includes all forms once it is isolated from the rest of the world. The context is that it receives no heat and is given an endless amount of time to settle into its own intrinsic qualities. That is in its own thermal equilibrium when it has entirely settled to the point where macroscopic change is no longer evident. This is not assumed that other types of the internal balance require it. For example, a body may achieve internal temperature equilibrium but not internal chemical equilibrium; glassware is an instance of this.
Consider an isolated system that is not yet in a condition of internal thermal equilibrium. It could be partitioned into two compartments isolated by nothing, no wall, in a fictitious thermodynamic action. The idea of energy exchanges as heat among the two subsystems might then be considered. The two subsystems would reach a practically steady state, and so be in thermal equilibrium with one another, a considerable period after the fictive partition procedure. Such an experience might be carried out in an infinite number of ways, using various fictive partitions. Screening subsystems from multiple partitions will end in subsystems that may be proved to be in thermal balance with each other.
As a result, an isolated system that is not initially with its own state of inner thermal equilibrium will almost always achieve a final state that may be considered one of internal thermal equilibrium if left for a long period. The final position is one of spatial uniformity of thermal homogeneity. A fundamental premise of classical thermodynamics is the presence of such states. The negative first law of thermodynamics is the name given to this postulate on rare occasions. Isolated quantum systems that are many-body localized but never establish internal thermal equilibrium are significant exceptions.
Thermal Contact:
A thermodynamic system is considered to be in thermal contact with another system in heat transfer and thermodynamics if it can interchange energy through the process of heat. Because real systems are always in thermal touch with their environment to some level, perfect thermal isolation is an idealization. Whenever two solid bodies come into touch, there is heat transfer resistance between them. Thermal contact conductance is the study of heat transfer between such bodies.
Significance of Thermal equilibrium in NEET exam:
The goal of NEET thermal equilibrium topics is to explain and present the most likely questions that will appear on the exam. These can be explained in simple terms using notes from experienced academics in the field, which are available on the Infinity Learn open website. Multiple-choice questions are simple to practice if students have a good understanding of the subjects presented throughout the program.
The present article on thermal equilibrium provides comprehensive information on the various types of thermodynamic laws. Examine the thorough notes carefully to make sure that you comprehend this topic, as it will aid your preparation for the NEET exam. You can also scribble some remarks on elastic energy to review during the exam.
Also read: Terminal velocity
Frequently asked questions
When is a system claimed to be thermally balanced but not thermodynamically balanced?
When two systems allow the transmission of heat energy but not matter or energy as work, they are considered to be in thermal equilibrium rather than thermodynamic equilibrium.
What are thermodynamics' zeroth laws?
When two systems are individually in equilibrium with a third system, the zeroth law of thermodynamics asserts that those two systems are likewise in thermal equilibrium with each other. TA = Tc and TB = Tc, then it is also possible that TA = TB
What is the significance of thermodynamic laws?
The rules of thermodynamics specify physical characteristics that characterize thermodynamic systems at thermal equilibrium, such as temperature, energy, and entropy.
What does bad work look like?
The work done by Kinetic Friction when pushing an object down the floor is negative.
For more visit NCERT Exemplar Solutions for Class 11 Physics Chapter 9 – Mechanical Properties of Solids