Table of Contents
Introduction:
The force of electricity EMF, for example, is a concept that most students are unfamiliar with. However, it is inextricably linked to the more familiar concept of voltage. They understand the difference between these two and what EMF means to provide us with the tools we need to solve many physics and electronics problems.
It will also introduce the concept of a battery’s internal resistance. EMF describes the voltage of a battery without accounting for internal resistance, which reduces the value. The electric potential produced by an electrochemical cell or by changing the magnetic field is called an electromotive force. EMF is an abbreviation for electromotive force.
The conversion of energy from one form to another is accomplished by using a generator or a battery. One terminal in these devices becomes positively charged, while the other becomes negatively charged.
As a result, work on a unit electric charge is characterized as an electromotive force. Electromotive force is used in the electromagnetic flowmeter based on Faraday’s law.
The electromotive force, or EMF, is the amount of energy supplied by a battery or cell per coulomb (Q) of charge passing through it. When there is no current flowing through the circuit, the magnitude of emf is equal to V (potential difference) across the cell terminals. Negative electromotive forces are possible. Consider where an inductor generates an EMF that opposes the incoming power. The produced EMF is then interpreted as unfavorable because the flow direction is opposite the real power. As a result, the electromotive force may be negative.
Overview:
The electromotive force of a cell, also known as the electromotive force of a cell, is the maximum potential difference between two cell electrodes. It is also known as the net voltage between the oxidation and reduction halves of the reaction. A cell’s EMF is primarily used to determine whether or not an electrochemical cell is galvanic.
An electrochemical cell is a device that uses a chemical reaction to generate electricity. It is essentially a device that converts chemical energy into electrical energy. An electrochemical cell requires a chemical reaction that involves the exchange of electrons to function. Such reactions are referred to as redox reactions.
The voltage of a cell defines it. Regardless of cell size, a specific cell type generates the same voltage. Given ideal operating conditions, the only thing that depends on cell voltage is the chemical composition of the cell.
Usually, the cell voltage will deviate from this ideal value due to various factors such as temperature differences, concentration changes, and so on. The Nernst equation, developed by Walther Nernst, can calculate the EMF value of a given cell if the cell’s standard cell potential is known.
EMF is calculated using two main equations. The basic definition is the number of joules of energy picked up by each coulomb of charge as it passes through the cell.
ε=E/Q
If we know the amount of charge passing through the cell and the resulting energy.
It is the simplest method for calculating the EMF. Instead, we can use a definition more akin to Ohm’s law, namely V = IR. So the formula is as follows:
ε=I(R+r)
ε=IR+Ir ε =V+Ir
This demonstrates that we can calculate the EMF by knowing the voltage across the terminals, the current flowing, and the cell’s internal resistance.
Potential difference and emf of a cell
EMF is the amount of energy (any form) converted into energy (electrical) per coulomb of charge. In contrast, the potential difference is the amount of energy (electrical) converted into other forms of energy per coulomb of charge. Sources of emf include cells, solar cells, batteries, generators, thermocouples, dynamos, etc.
A terminal potential difference in a cell
The terminal voltage is the potential difference across the terminals when the circuit is turned on. On the other hand, EMF is the highest potential difference that a cell or generator may produce when no current flows through it. When the terminal voltage is measured using a voltmeter, the electromotive force is calculated using a potentiometer.
Because of the potential drop caused by the current passing through the cell’s internal resistance, the terminal voltage is always lower than the EMF. Terminal voltage does not retain constancy, but EMF is a cell’s characteristic constant. A cell’s terminal potential difference is the potential difference between its two electrodes in a closed circuit.
When current is drawn from a cell, the terminal potential difference is less than the emf of the cell (i.e., during discharging of the cell), when a current flows, the internal resistance r of a voltage source influences the output voltage.
The terminal voltage V of a device is given by V = emf Ir, where I is the electric current and is positive when flowing away from the positive terminal of the voltage source.
Difference between cell potential and emf:
| Electromotive force (emf) | Potential Difference |
| E.m.f denotes the energy supplied by the cell to the unit charge. | The energy dissipated as the unit charge passes through the components is the potential difference. |
| The cause is e.m.f. | The effect is the potential difference. |
| Even when no current is drawn through the battery, the emf is present. | In the absence of current, the potential difference across the conductor is zero. |
| It is never less than a potential difference. | It is never less than emf. |
| It transmits current both within and without the cell. | The potential difference in current transfer between two points in the cell. |
Relation between internal resistance emf and terminal potential difference of a cell:
All voltage sources have two basic components: an internal resistance r and an electrical energy source with an electromotive force characteristic (emf). So, the emf is the potential difference between a source when no current is flowing through it and when current is flowing through it. The internal resistance r of the voltage source, on the other hand, influences the output voltage when the current passes through it. Because of the voltage drop across the internal resistance of the device, the terminal voltage of a battery is smaller than the electromotive force(emf) when it is discharging. If current flows “backward” from positive to negative poles in a battery, the terminal voltage can be estimated using the battery’s emf, and the voltage drop across the internal resistance.
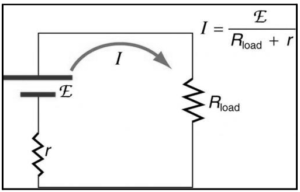
Let E be the emf and V be the terminal voltage. With a load R, the current flowing I, and internal resistance r, the equation can be written as –
I=E/R+r
The potential difference between the terminal voltages is computed as follows:
V=I.R
=ER/R+r
Frequently Asked Question (FAQs):
What is the difference between voltage and potential difference?
The electrical potential V of a position in the electrical field is such that the electric potential energy required to place a particle of charge q at that position is the product of the particle's charge q and the position's potential V(t).
What does it indicate when two points have a potential difference of 1 V?
The potential difference between the two points is 1V if 1J of work is necessary to transport a charge of 1C from one point to another.
What Is the Meaning of a Battery's EMF, and What Is the Relationship Between EMF and Voltage?
Electromotive force is the amount of work done in the energy transformation (or conversion) and the amount of electricity that travels through the electrical source or generator. The electromotive force is measured in volts (EMF). The source's voltage is not the same as the source's emf. Voltage is defined as the potential difference between the two electrode potentials of a battery under any conditions. EMF is an important topic in physics because it has connections to more complex topics and their applications.









