Table of Contents
A nucleophilic addition reaction is a chemical addition reaction in which a nucleophile forms a sigma bond with an electron-deficient species. These reactions are considered veritably important in organic chemistry since they enable the conversion of carboxyl groups into a variety of functional groups. Generally, nucleophilic addition reactions of carbonyl composites can be broken down into the following three ways.
The electrophilic carbonyl carbon forms a sigma bond with the nucleophile.
The carbon-oxygen pi bond is now broken, forming an alkoxide intermediate (the bond brace of electrons are transferred to the oxygen snippet).
The posterior protonation of the alkoxide yields the alcohol outgrowth.
The carbon-oxygen double bond is directly attacked by strong nucleophiles to give rise to the alkoxide. Still, when weak nucleophiles are used, the carbonyl group must be actuated with the help of an acid catalyst for the nucleophilic addition reaction to occur.
The given carbonyl group usually has a coplanar structure and therefore, its carbon is usually sp2 hybridized. Still, the attack of the nucleophile on the C =O group results in the breakage of the pi bond. Hence, The carbonyl carbon in the very next step is sp3 hybridized and therefore it forms a sigma bond with the nucleophile.
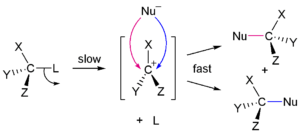
Nucleophilic addition reaction examples
Nucleophilic Addition reaction with Carbonyl Composites
In carbonyl composites, the carbon-oxygen bond is polar. Owing to the fairly advanced electronegativity of the oxygen snippet, the electron viscosity is advanced near the oxygen snippet. This leads to the generation of a partial negative charge on the oxygen snippet and a partial positive charge on the carbon snippet.
As given the carbonyl carbon holds a little partial positive charge, the carbonyl carbon then behaves as an electrophile. The partial negative charge on the oxygen snippet can be stabilized via the preface of an acidic group. The proton bestowed by the acid binds with the carbonyl oxygen snippet and neutralizes the negative charge.
Aldehydes are fairly more reactive towards nucleophilic addition reactions when compared to ketones. This is because the secondary carbocations formed by ketones are stabilized by the conterminous R groups. The primary carbocations formed by aldehydes are less stable than the secondary carbocations formed by ketones and are, thus, more susceptible to nucleophilic attacks.
Nucleophilic Addition reactions with Hydrogen Cyanide
The nucleophilic addition reaction between hydrogen cyanide (HCN) and carbonyl composites ( generally aldehydes and ketones) results in the conformation of cyanohydrins. Base catalysts are frequently used to increase the rate of the reaction. The cyanide anion (CN –) acts as an important nucleophile and attacks the carbonyl carbon to form a new sigma bond
The given polar nature of the C = O bond usually makes the carbonyl carbon electrophilic in nature. The cyanide anion executes a nucleophilic attack on the carbonyl carbon, performing in the conformation of an intermediate. This intermediate is now protonated to get the cyanohydrin product.
Nucleophilic Additions reaction with Monohydric Alcohols
Aldehydes and ketones suffer nucleophilic addition reactions with monohydric alcohols to yield hemiacetals. Upon further reaction with another patch of the alcohol, an acetal is attained. Alcohols are usually weak nucleophiles, hence this reaction often requires acid in the form of a catalyst for the activation of the carbonyl group towards the nucleophilic attack.
Since the hemiacetals can suffer hydrolysis to yield the reactants (the alcohol and carbonyl emulsion), the water formed during the reaction must be removed. In the given reaction, the carbonyl oxygen is often protonated before some nucleophilic attack is carried out by the given alcohol. The nucleophilic alcohol in the next step is usually deprotonated just to form the hemiacetal. This reaction can be repeated to gain the acetal.
Nucleophilic Addition reaction with Grignard Reagents
The polar nature of Grignard reagents ( general formula R-Mg-X) attributes a partial negative charge to the carbon snippet.
Primary alcohols are formed when
formaldehyde is used.
→ Other aldehydes yield secondary alcohols upon replying with Grignard reagents.
→ The nucleophilic addition reactions between ketones and Grignard reagents yield tertiary alcohols.
The general medium of these reactions involves the attack of the nucleophilic carbon ( belonging to R-Mg-X) on the carbonyl carbon. A simple acid workup of the performing alkoxide yields the corresponding alcohol.
Nucleophilic Addition reaction with Primary Amines
The reaction between primary amines and aldehydes/ ketones yields imine derivations along with water.
Originally, the nucleophilic nitrogen belonging to the amine attacks the carbonyl carbon. Now a proton is transferred from the amine to the oxygen snippet. In the coming step of this nucleophilic addition reaction, The OH group is further protonated and water is removed.
The carbon snippet now forms a double bond with the nitrogen belonging to the amine. This nitrogen is now deprotonated to get the needed imine product.
Mechanism of Nucleophilic Addition Reaction
A nucleophile acts on the polar carbonyl’s electrophilic carbon snippet vertically to the orbital demonstration sp2 hybridization of the carbonyl carbon structure.
Still, on the attack of the nucleophile, the hybridization of the carbon snippet changes from sp2 hybridization to sp3 hybridization thereby forming a tetrahedral alkoxide intermediate complex. This intermediate complex will take a proton from the response medium to produce an electrically neutral emulsion. Hence, the response results in the addition of nucleophile and hydrogen in the carbon-oxygen double bond.
Aldehyde and ketones demonstrate polar nature. Also, these composites have an advanced boiling point in comparison to hydrocarbons. Still, aldehydes and ketones have lower boiling points in comparison to alcohol. The numerous responses involving aldehydes and ketones are sufficient for different conflation responses.
Still, the maturity of characteristic responses of aldehydes and ketones involves a nucleophilic addition to the carbonyl group.
Nucleophilic Addition Reaction of Aldehyde and Ketone
Aldehydes are largely reactive and readily suffer nucleophilic addition responses compared to ketones. Aldehydes demonstrate numerous favourable equilibrium constants for additional responses than ketones due to the electronic and steric effects. Aldehydes present more favourable equilibrium constants for fresh responses than ketones due to electronic and steric results.
Concerning the ketones case, two large substituents are contained in the ketone structure, which causes steric interference when the nucleophile approaches the carbonyl carbon. After all, aldehydes comprise one cover, and therefore the steric interference to the approaching nucleophile is comparatively less. In discrepancy, electronically aldehydes parade better reactivity than ketone.
It’s because ketones contain two alkyl groups that reduce the carbonyl carbon tittles’ electrophilicity rather than aldehydes.
The rate-determining step with respect to the base-catalyzed nucleophilic addition response and the acid-catalyzed nucleophilic addition response is the step, wherein the nucleophile works on carbonyl carbon.
Also, the protonation process happens in carbonyl oxygen after a nucleophilic addition step in the case of acid catalysis conditions. The carbocation character of the carbonyl structure increases due to protonation and therefore makes it further electrophilic
FAQs
1. Explain the nucleophilic reaction with primary amines.
Ans. The response between the primary amines and aldehydes or ketones generates imine derivations along with water. The same can be seen below. Originally, the nucleophilic nitrogen of the amine attacks carbonyl carbon. The carbon-oxygen double bond is therefore broken, and a new sigma bond of carbon-nitrogen is formed.
The proton is now being transferred from the amine to the oxygen snippet. In the coming step of this nucleophilic addition response, the OH group is farther protonated, and the water is removed. Now, the carbon snippet forms a double bond with the nitrogen that belongs to the amine. This nitrogen is now deprotonated to go the necessary imine product.
2. Why are nucleophilic addition reactions important?
Ans. Nucleophilic addition responses is an important content in chemistry so is to understand them. Nucleophilic addition response is a chemical addition response in which a nucleophile establishes a sigma bond with an electron-deficient patch and thus it’s known as a nucleophilic addition response.
These types of responses are veritably pivotal in the section of organic chemistry as they allow carbonyl groups to be converted into a variety of functional groups also The carbon electrons are incompletely positive in charge, whereas the oxygen electrons are incompletely negative and because of steric and electrical factors, aldehydes are constantly more reactive toward nucleophilic reserves rather than ketones.







