Table of Contents
Phosphine is a chemical that is classified as an organophosphorus compound. The structure of phosphine is trigonal pyramidal. Metal phosphides react with water or HCl to create phosphine. It’s a colourless gas that’s particularly toxic. The concentration of this chemical in the atmosphere fluctuates. It plays an essential role in the phosphorus biochemical cycle. In nature, phosphine may be found in human blood, urine, and saliva.
When aluminium, calcium, magnesium, or zinc phosphide pellets are exposed to water vapour, phosphine gas is formed. Aluminium phosphide is largely employed in the treatment of grain and tobacco warehouses. Its pellet composition lends itself to application in the treatment of shipping containers.
Phosphine is an insecticide that is used to fumigate grains, animal feed, and leaf-stored tobacco.
In humans, acute (short-term) phosphine inhalation exposure can produce headaches, dizziness, exhaustion, sleepiness, searing substernal discomfort, nausea, vomiting, cough, difficulty breathing, chest tightness, pulmonary irritation, pulmonary oedema, and tremors. Convulsions may occur after a brief period of apparent recovery. Chronic (long-term) occupational phosphine exposure may result in the nasal cavity and throat inflammation, weakness, dizziness, nausea, gastrointestinal, cardiorespiratory, and central nervous system symptomology, jaundice, liver consequences, and increased bone density.
Overview:
Phosphine, denoted by the formula PH3, is a phosphorus hydride. Phosphine is a dangerous, flammable gas. Because of its vital industrial applications, it is extensively manufactured in businesses and laboratories.
Phosphine is a chemical that is classified as an organophosphorus compound. Philippe Gengembre discovered this chemical for the first time in 1783. He made phosphine by heating phosphorous in an aqueous potassium carbonate solution. The concentration of this chemical in the atmosphere fluctuates. It plays an essential role in the phosphorus biochemical cycle. In nature, phosphine may be found in human blood, urine, and saliva.
Phosphine is found in extremely low and very variable amounts in the Earth’s atmosphere. It has the potential to make a large contribution to the global phosphorus biogeochemical cycle.
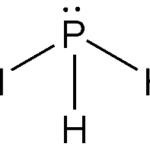
Structure of Phosphine:
The structure of phosphine is trigonal pyramidal. The lone pair of electrons on phosphorus changes the symmetry of the molecule. Phosphorus undergoes sp3 hybridization. There are three bond pairs and one lone pair of electrons. The H–P–H bond has a 93.5°angle, while the P–H bond has a length of 1.42 pm.
Preparation of Phosphine:
1. Metal phosphides hydrolyze with calcium phosphide- Metal phosphides react with water or HCl to create phosphine.
Ca3P2+6H2O→2PH3+3Ca(OH)2
Ca3P2+6HCl→2PH3+3CaCl2
2. Pure phosphine is synthesized from phosphorous acid by the breakdown of H3PO3.
4H3PO3→3H3PO4+PH3
3. Heat white phosphorus in a concentrated NaOH solution in an inert CO2 atmosphere to create PH3.
P4+3NaOH+3H2O→PH3+3NaH2PO2
Purification of Phosphine:
Pure phosphine is a non-flammable liquid. It becomes flammable due to the presence of P2H4 or P4 vapours. By absorbing phosphine in HI and turning it to PH4l, phosphine is purified. When this is combined with potassium hydroxide, phosphine is formed.
PH4I+KOH→PH3+KI+H2O
Physical Properties of Phosphine:
- It’s a colourless gas that’s particularly toxic.
- It smells strongly of rotting fish.
- It is only marginally soluble in water.
- Its boiling point is –87.7 degrees Celsius.
- As previously established, pure phosphorus is non-flammable. Despite this, it is flammable due to the presence of P4 vapour. Phosphorus may catch fire when it comes into contact with oxidizing chemicals such as HNO3, chlorine, or bromine vapours. It can catch fire even if just a tiny amount of these oxidizing substances is present.
Chemical Properties of Phosphine:
- Phosphine is a Lewis base, just like ammonia. It creates phosphonium compounds when it combines with HI, HBr, and HCl.
PH3+HI→PH4I
PH3+HBr→PH4Br
PH3+HCl→PH4Cl
- Phosphorus pentoxide and water are produced when phosphorus is burnt.
2PH3+4O2→P2O5+3H2O
- Reaction with chlorine
PH3+4Cl2 →PCl5+3HCl
- It will not ignite under normal conditions. When heated, it bursts with flame, producing phosphoric acid.
- It bursts dramatically when exposed to oxidizing chemicals such as HNO3, Cl2, and Br2.
- In the presence of light, PH3 in water decomposes to create red P and H2O.
PH3(H2O)→P(red)+H2O
- When phosphine is absorbed in copper sulfate or mercuric chloride, copper phosphide and mercuric phosphide are formed.
3CuSO4+2PH3→Cu3P2+3H2SO4
3HgCl2+2PH3→Hg3P2+6HCl
Uses of Phosphine:
- Phosphine is a chemical that is utilized in the production of metallic phosphides.
- Phosphorus is present in Holme’s signal and Smokescreens.
Holme’s indication: Because phosphine spontaneously burns, it is a potential candidate for use in Holme’s signal. When a ship needs help, calcium carbide and calcium phosphide-filled containers are perforated and hurled into the ocean. As a result of the contact with water, acetylene and phosphine gases are generated. When these gases burn in the air, they send a signal.
The screen of smoke: When calcium phosphide is soaked in water, a significant amount of phosphine is produced, resulting in a smokescreen. During the war, this was used to hide troops from the enemy.
- Phosphine fumigants are used in rodent and insect control formulations.
Chlorodiphenylphosphine:
Chlorodiphenylphosphine abbreviated Ph2PCl is an organophosphorus chemical having the formula (C6H5)2PCl. It is a colourless oily liquid with a strong odour that has been characterized as garlic-like and is perceptible even at low concentrations (ppb). It is an effective reagent for introducing the Ph2P group into molecules, which includes a wide range of ligands. Ph2PCl, like other halophosphines, is reactive with various nucleophiles, including water, and is quickly oxidized even by air.
Chlorodiphenylphosphine, like other chlorophosphines, is utilized in the manufacture of phosphines.
Ph2PCl-derived phosphines are further developed and used as pesticides (such as EPN), plastic stabilizers (Sandostab P-EPQ), various halogen compound catalysts, flame retardants (cyclic phosphinocarboxylic anhydride), and UV-hardening paint systems (used in dental materials), making Ph2PCl an important intermediate in the industrial world.
FAQs
Q. What are the characteristics of phosphine?
ANS:
- Phosphine is a colourless and very poisonous gas.
- It has a strong rotten-fish odour.
- It is insoluble in water.
Q. Is phosphine a non-polar element?
ANS: No. Although phosphine is a polar element, it has non-polar molecular bonding. This is clear from the chemical formula PH3 (3 Hydrogen bonds with a lone pair of electrons).
Q. How is phosphine purified?
ANS: Phosphine is refined by absorbing impurities in HI, which produces PH4I, which is then treated with potassium hydroxide to produce phosphine.
PH4I+KOH→KI+H2O+PH3









