Table of Contents
Introduction
Light scattering by particles in a colloid or very fine suspension causes the Tyndall effect. It is similar to Rayleigh scattering in that the intensity of scattered light is inversely proportional to the fourth power of the wavelength, so blue light is scattered much more strongly than red light. In everyday life, an example is a blue color seen occasionally in the smoke emitted by motorcycles, particularly two-stroke machines, where the particles are provided by the burnt engine oil. Longer wavelengths are more transmitted by the Tyndall effect, while shorter wavelengths are more diffusely reflected by scattering. When the light-scattering particulate matter is dispersed in an otherwise light-transmitting medium, the Tyndall effect is observed when the diameter of an individual particle is between 40 and 900 nm, i.e. slightly below or near the wavelengths of visible light (400–750 nm). It is especially useful for colloidal mixtures and fine suspensions; for example, the Tyndall effect is used in nephelometers to determine particle size and density in aerosols and other colloidal matter. It is named after the 19th-century physicist John Tyndall, who conducted extensive research on the phenomenon.
The scattering of light as a light beam passes through a colloid is known as the Tyndall effect. The beam may be seen because individual suspension particles scatter and reflect light.
The amount of scattering is determined by the frequency of the light and particle density. The Tyndall effect, like Rayleigh scattering, scatters blue light more strongly than red light. Light with longer wavelengths is transmitted, while light with shorter wavelengths is reflected through scattering.
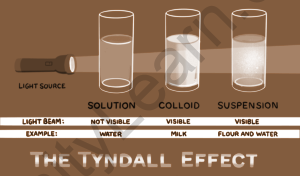
The size of the particles distinguishes a colloid from a real solution. The particles in a mixture must be between 1-1000 nanometers in diameter in order for it to be classified as a colloid.
Overview
The Tyndall effect is the phenomenon that occurs when particles in a colloid scatter light beams directed at them. This effect can be seen in all colloidal solutions including certain very tiny suspensions. As a result, it may be used to tell if a solution is a colloid or not. The density of colloidal particles, as well as the frequency of incident light, influence the intensity of scattered light. When a light beam passes through a colloid, the colloidal particles in the solution prevent the beam from passing completely through. Light is scattered when it collides with colloidal particles (it deviates from its normal trajectory, which is a straight line). This scattering reveals the path of the light beam, as shown below.
The Tyndall effect describes the dispersion of light as a light beam passes through a colloid. When light beams are focused on particles in a colloid, the Tyndall effect occurs. This effect can be observed in all colloidal solutions, including very small suspensions. As a result, it can be used to determine whether a given solution is a colloid. The density and frequency of the suspended particles influence the intensity of the dispersed light. Like Rayleigh scattering, the Tyndall effect scatters blue light more than red light. When a light beam passes through a colloid, colloidal particles in the solution prevent the beam from passing completely through.
The amount of melanin in one of the iris layers is the primary difference between brown, blue, and black irises. In comparison to a black iris, the layer of a blue iris contains less melanin, making it transparent. The light that strikes this transparent layer is dispersed due to the Tyndall effect. Because blue light has a shorter wavelength than red light, it is more dispersed. Irises absorb light that has not been scattered from a deeper layer. Because the majority of the light dispersed is blue, the iris is blue. Light scattering is involved in a variety of processes. Mie scattering and Rayleigh scattering are two examples of this. The color of a clear sky is blue because the light is scattered by air particles, which is an example of Rayleigh scattering. However, when the sky is overcast, light scattering is caused by the comparatively large cloud droplets, which is an example of Mie scattering.
Tyndall Effect
Tyndall effect refers to the phenomenon by which colloidal particles scatter light. When light passes through a colloid, it is scattered by the larger colloidal particles, causing the beam to become visible. This is known as the Tyndall effect.
Tyndall effect diagram
John Tyndall, a physicist, is commemorated by the name of this phenomenon. It specifies how various types of mixtures react to the passage of light focused on them. To grasp the concept of this effect, we must first determine which wavelengths of light are reflected and which are transmitted. Light particles with shorter wavelengths are scattered, while those with longer wavelengths can pass through.
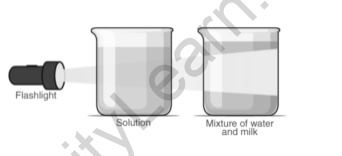
When we shine a flashlight into these beakers, the light rays pass through the flour and water mixture. Despite the fact that the other mixture is a clear solution, light cannot pass through it. This is due to the fact that flour particles are larger in size and have a larger surface area than red sugar particles. Light particles are suspended by them due to their surface area, resulting in the Tyndall effect.
When a light beam is focused on a colloid and a solution, light particles become suspended as they pass through the colloid particles. In the case of a solution, however, the light particles are not prevented from sinking.
Properties of colloids
Colloids are heterogeneous mixes of two substances in which minute particles of one component are scattered in another substance, according to chemistry. The dispersed phase is a substance whose minute particles are suspended in another substance, while the dispersion medium is the substance in which it is suspended. In fog, for example, the dispersed phase is water (liquid), and the dispersion medium is various gases. Because dispersed phase particles in colloids are so minute, we can’t see them with our naked eyes.
The qualities of colloids are as follows.
- It is a mixture that is diverse.
- Colloidal particles have an extremely small size. The size of their particles ranges from 1 to 1000 nanometers.
- The Tyndall effect is demonstrated. It scatters the light beam and reflects its route across itself.
- When they are left alone for an extended period of time, they do not calm down. It implies that colloidal solutions are extremely stable.
- They do not calm down when left alone for an extended period of time. This indicates that colloidal solutions are quite stable.
- The filtration process cannot separate them. Centrifugation can be used to separate them.
- Brownian movement is observed in colloidal particles.
Finally, it is a phenomenon that is observed when light passes through colloidal solutions. The Tyndall effect is used in commercial and laboratory applications to measure the particle size of aerosols because light scatters.
FAQs
What causes the Tyndall effect?
The Tyndall effect occurs as a result of incident light reflection from the surface of the particles, reflection from the interior walls of the particles, and refraction and diffraction as it passes through the particles.
What are the characteristics of the Tyndall effect?
The Tyndall effect occurs when hyaluronic acid fillers are injected very close to the surface, resulting in bluish discoloration of the skin of the eyelids. As a result, the tear trough has an unnatural puffiness and an irregular contour.
What exactly do colloids explain?
Colloids are mixtures that contain one or more chemicals that are distributed as relatively large solid particles or liquid droplets throughout a solid, liquid, or gaseous medium. The particles in a colloid are typically electrically charged, and they remain distributed rather than settling due to gravity.





