Table of Contents
A chemical bond is a long-lasting attraction between atoms, ions, or molecules that allows chemical compounds to form. The electrostatic force of attraction between oppositely charged ions forms ionic bonds, whereas the sharing of electrons forms covalent bonds. Strong bonds, such as covalent, ionic, and metallic bindings, are “primary bonds,” whereas “weak bonds,” such as dipole-dipole interactions, the London dispersion force, and hydrogen bonding, are “secondary bonds.”. Because opposite charges attract each other through a simple electromagnetic force, the negatively charged electrons orbiting the nucleus and the positively charged protons in the nucleus attract each other. The chemical bond is formed by this attraction. Because of the matter-wave nature of electrons and their lower mass, they must occupy a much larger volume than nuclei, and this volume occupied by the electrons keeps the atomic nuclei in a bond relatively far apart, in comparison to the size of the nuclei themselves. Strong chemical bonding is generally associated with the sharing or transfer of electrons between the atoms involved. Chemical bonds hold together the atoms in molecules, crystals, metals, and diatomic gases—indeed, most of the physical environment around us—and dictate the structure and bulk properties of matter.
The valence and bonding preferences of a solid’s constituent atoms can usually be used to predict its properties. The four major types of bonding are discussed here: ionic, covalent, metallic, and molecular. Another important category in a few crystals is hydrogen-bonded solids, such as ice. Many solids have a single bonding type, whereas others have a mixture of types, such as covalent and metallic or covalent and ionic.
Overview
Chemical bonding is the attractive force that holds various constituents (atoms, ions, etc.) together and stabilizes them through the overall loss of energy. As a result, the strength of chemical bonds between constituents determines the stability of chemical compounds; the stronger the bonding between constituents, the more stable the final complex. If the chemical interaction between the ingredients is weak, the new product will be unstable and will quickly undergo another reaction to form a more stable chemical complex (containing stronger bonds). In order to achieve stability, atoms try to shed energy. A force is exerted on the first when one kind of matter interacts with another. The energy falls when the forces of nature are attractive. When nature’s powers are repellent, the energy level rises.
The attractive force that holds two atoms together is known as the chemical bond. When substances participate in chemical bonding and produce compounds, the type of chemical bonds contained in the resulting compound can be used to determine its stability. The chemical bonds created have varying strengths and qualities. Atoms or molecules form four different types of chemical bonds to produce compounds. Chemical bonds of this type include:
- Covalent Bonds
- Ionic Bonds
- Bonds of Hydrogen
- Polar Bonds
Chemical bonds of this type are formed by the loss, gain, or sharing of electrons between two atoms/molecules.
Types of chemical bonds
Regardless of the common times of solid, liquid, and gas – for example, water exists as ice, liquid water, and vaporous steam – various states, such as plasma, Bose-Einstein condensates, fermionic condensates, and quark-gluon plasma, are possible. Aside from that, it is classified as pure substances and mixtures.
Chemical bonding is the process of joining two or more atoms through electron redistribution, which results in each atom achieving a stable electronic state. To ensure security, each atom completes its duplet or octet by obtaining the nearest tolerable gas electronic arrangement. It is typically accomplished through the formation of composite connections between particles.
A particle can form chemical bonds in three ways:
At least one electron is transferred to a different atom.
Getting at least one electron from another atom.
By transferring an electron from one atom to another.
Atoms tend to arrange themselves in the most stable patterns possible, which means that their outermost electron orbits tend to be completed or filled. They collaborate with other atoms to accomplish this. A chemical bond is a force that holds atoms together in collections known as molecules. Chemical bonds are classified into two main types and a few secondary types.
Types of covalent bond
A chemical bond created by the exchange of electron sets between particles is known as a covalent bond. Covalent holding is the stable balance of appealing and disgusting powers between particles when they share electrons, and these electron sets are called shared matching or holding sets. For some mixes, electron sharing allows each particle to achieve something akin to a complete valence shell, which corresponds to a stable electronic state. In natural science, covalent bonds are far more common than ionic bonds. Covalent bonds, also known as molecular bonds, are established between two atoms or ions in which the electron pairs are shared between them. The attraction or repulsion between two atoms is known as covalent bonding (when they share an electron pair or bonding pair). The shared pair of electrons now extends around the nuclei of the atoms, resulting in the formation of a molecule.
The number of shared electron pairs in a covalent connection can be categorized into three types. The following are examples of covalent bonds:
- Single Covalent Bond
- Double Covalent Bond
- Triple Covalent Bond
Single Covalent Bond
When only one pair of electrons is shared by the two participating atoms, the bond is referred to as a single covalent bond. A single dash is used to indicate it (-). This type of covalent bond has a lower density and is weaker than a double or triple bond, despite being the most stable.
The HCL molecule, for example, contains one Hydrogen atom with one valence electron and one Chlorine atom with seven valence electrons. In this case, a single bond between hydrogen and chlorine is formed by sharing one electron, completing the octet of one HCL molecule.
Double Covalent Bond
A double bond is formed when two pairs of electrons are shared by the two participating atoms. It’s denoted by two dashes (=). Double covalent bonds are substantially more powerful than single covalent bonds, but they are also less stable. A carbon dioxide molecule, for example, contains one carbon atom with six valence electrons and two oxygen atoms with four valence electrons.
Triple Bonding
A triple bond is formed when three pairs of electrons are shared by the two participating atoms. Three dashes () represent triple covalent bonds. These are the covalent bonds that are the least stable.
In the formation of a nitrogen molecule, each nitrogen atom with five valence electrons exchanges three electrons with each other to form three electron pairs that complete the octet. As a result, the two nitrogen atoms create a triple bond.
Ionic and covalent bonds
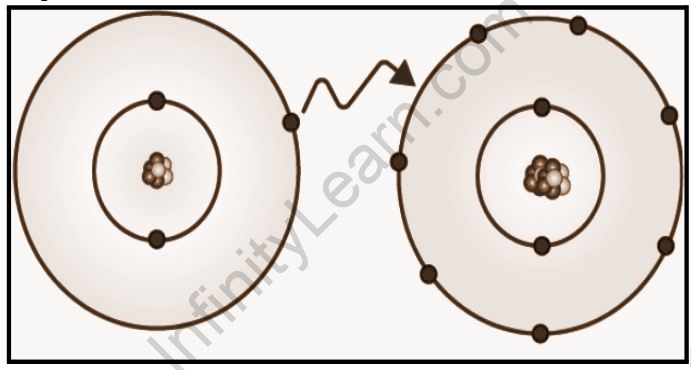
Ionic bonding is a type of chemical bonding that involves the transfer of electrons from one atom or molecule to another. An atom loses an electron in this situation, which is then gained by another atom. When an electron transfer takes place, one of the atoms gains a negative charge and is referred to as an anion. The other atom, referred to as the cation, gains a positive charge. The charge differential between the two atoms determines the strength of the ionic connection; the bigger the charge disparity between the cation and the anion, the stronger the ionic bond.
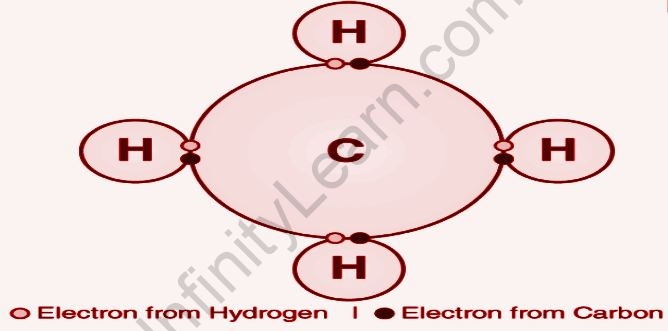
The exchange of electrons between atoms is referred to as a covalent bond. In carbon compounds, this sort of chemical bonding is frequent (also known as organic compounds). The two atoms’ shared electrons now stretch around their nucleus, culminating in the formation of a molecule.
Covalent bonding can be polar or nonpolar in nature. Because the more electronegative atom draws the electron pair closer to itself and away from the less electronegative atom, electrons are shared unequally in Polar Covalent chemical bonding. A good example of a polar molecule is water. There is a charge difference in different parts of the atom due to the irregular spacing of electrons between the atoms. The molecule is partially positively charged on one end and partially negatively charged on the other.
Also read: Polar Covalent Bond
FAQs
What are the four different types of chemical bonding?
Ionic bonds, covalent bonds, hydrogen bonds, and van der Waals interactions are the four types of chemical bonds required for life to exist. These different types of bonds are required to play various roles in biochemical interactions.
What are the strongest chemical bonds?
The most powerful chemical bond is the covalent bond. They formed between two atoms due to the mutual sharing of electrons. Because hydrogen and oxygen atoms exchange electrons, water is a classic example of a covalent bond.









