Table of Contents
pH of Substances
Table of Contents
- Indicators
- Universal Indicator and the pH Scale
- Summary
- Did You Know?
- What’s Next?
In the previous segment of the chapter ‘Acids, Bases and Salts’, we got introduced to the pH of substances. In this segment, let us learn to measure the pH of substances in real life.
What are Indicators?
Substances which show the acidic or basic behaviour of other substances are called Indicators. The common types of indicators are:
- Natural – Indicators obtained from natural sources are called Natural indicators. For example, litmus and turmeric.
Litmus is obtained from lichens. Acids turn blue litmus paper red and bases turn red litmus paper blue.

Litmus and Turmeric
- Olfactory – Substances which change their smell when mixed with acid or base are called
Olfactory indicators.
For example, onion and vanilla.
Paste or juice of onion loses its smell when added with bases but does not change its smell with acids. The smell of vanilla vanishes with bases but does not vanish with acids.

Onion and Vanilla
- Synthetic – Indicators synthesized in the laboratory are called Synthetic indicators.
For example, phenolphthalein and methyl orange. They give the acidic or basic character of a solution.
Phenolphthalein is a colourless liquid and remains colourless with acid but turns pink with a base. Methyl orange is originally orange in colour but turns red with acid and yellow with base.


Phenolphthalein and Methyl orange
- Universal – A universal indicator shows different colours over the range of pH values from 1 to 14 for a given solution.
It is found in the form of strips and solutions. It gives the strength of acids or bases.
pH values from 0 – 6 show acidic nature, pH values from 8 – 14 show basic behaviour and the pH of 7 shows neutral behaviour.
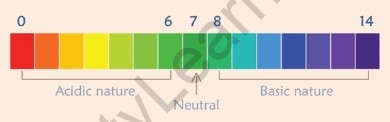
Universal Indicator
Which colours are shown by different substances in the universal indicator?



